Minhua Chu, Managing Partner at TransitionValue Partner, shared on X:
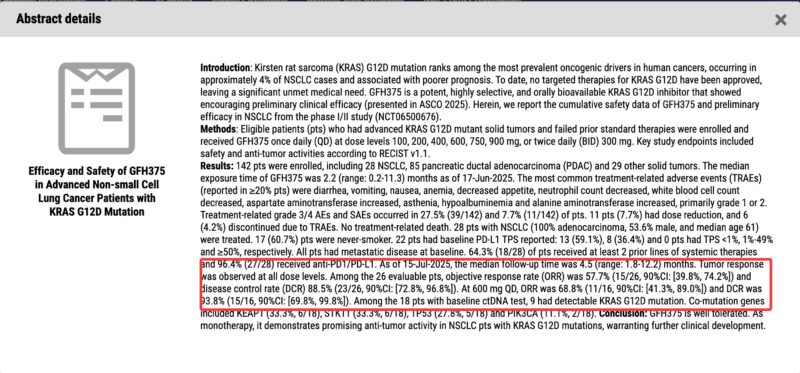
“GenFleet Therapeutics announced that the latest clinical trial data for its small-molecule KRAS G12D inhibitor, GFH375, as a monotherapy for NSCLC patients has been selected for WCLC2025 LBA and a mini-oral presentation.
The Phase 1/2 study demonstrated that GFH375 has an overall favorable safety profile in patients with KRAS G12D-mutant solid tumors, with notable efficacy and good safety/tolerability in NSCLC patients.
As of July 15 this year, the median follow-up time for 28 NSCLC patients was 4.5 months, with tumor responses observed across multiple dose groups.
Among 26 evaluable NSCLC patients, the objective response rate (ORR) was 57.7%, and the disease control rate (DCR) was 88.5%. In the 600 mg QD, the ORR was 68.8%, and the DCR was 93.8%.”
More posts featuring Minhua Chu.
