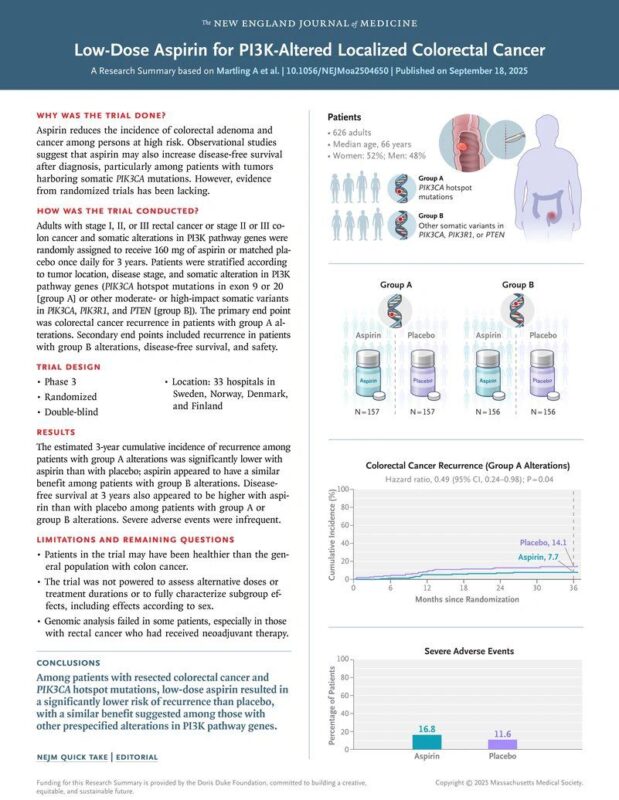
Amol Akhade, Senior Consultant at Fortis Hospitals Mumbai, shared a post on LinkedIn about a paper by Anna Martling et al. published in NEJM:
“NEJM 2025: ALASCCA Trial – Aspirin as Precision Adjuvant in Colorectal Cancer?
The ALASCCA trial (Martling et al., NEJM 2025) is a landmark step in repurposing a cheap, globally accessible drug — aspirin — within the framework of precision oncology.
Trial design:
6,397 patients screened → 2,980 sequenced → 1,103 (37%) harbored PI3K-pathway alterations. Hotspot PIK3CA exon 9/20 mutations: 17% Other PI3K/PTEN/PIK3R1 variants: 20%
626 randomized to aspirin 160 mg vs placebo for 3 years, double-blind, registry-based.
Results:
In hotspot (Group A): recurrence 7.7% vs 14.1% (HR 0.49, p=0.04). In other PI3K variants (Group B): recurrence 7.7% vs 16.8% (HR 0.42).
Disease-free survival trended positive, but overall survival benefit not shown yet.
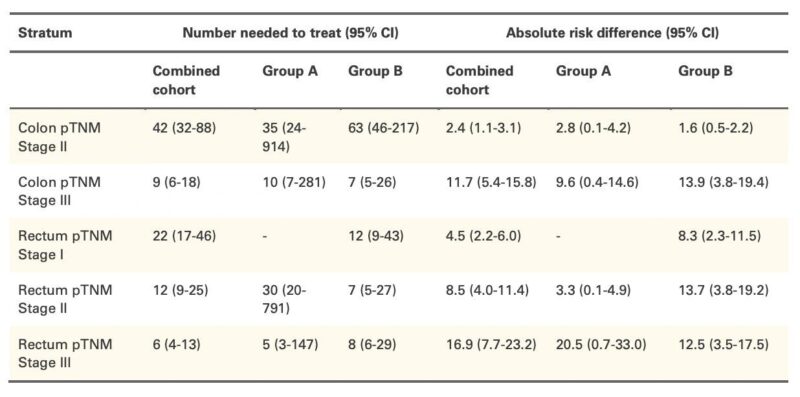
Number Needed to Treat (NNT):
Colon stage II → weak (NNT 42, ARD ~2%). Colon stage III → strong (NNT 9, ARD ~12%). Rectum stage III → best (NNT 6, ARD ~17–20%). Stage I/II rectum → modest, inconsistent benefit.
Safety:
Severe AEs: 16.8% with aspirin vs 11.6% with placebo. Most common: GI bleeding, thromboembolism, post-op complications. One aspirin-related death reported.
Critical view:
Strengths: biomarker-driven design, pragmatic registry-based RCT, global relevance of a low-cost intervention.
Limitations: borderline statistical significance (p=0.04 in Group A), wide confidence intervals, subgroup heterogeneity (sometimes non-hotspot mutations outperformed hotspots), and no OS benefit yet.
Clinical implication: Most compelling signal: stage III rectal and colon cancers. Stage II benefit marginal. Aspirin may join the armamentarium in high-risk, biomarker-selected subgroups, but universal use is premature.
Editorial take (Goldberg and Meyerhardt, NEJM):
They argue that biomarker-guided aspirin could mark long-awaited progress in adjuvant CRC therapy, similar to MSI-high immunotherapy (ATOMIC trial). But careful balancing of recurrence reduction vs bleeding risk is essential.
Bottom line:
The ALASCCA trial demonstrates the promise of repurposed, low-cost drugs guided by genomics. Aspirin halves recurrence in PI3K-altered CRC, but the benefit is heterogeneous and not yet practice-changing.
Future pooled analyses and replication will decide if aspirin truly becomes a standard precision adjuvant.”
Title: Low-Dose Aspirin for PI3K-Altered Localized Colorectal Cancer
Journal: NEJM
Authors: Anna Martling, Ida Hed Myrberg, Mef Nilbert, Henrik Grönberg, Fredrik Granath, Martin Eklund, Tom Öresland, Lene H. Iversen, Carola Haapamäki, Martin Janson, Karin Westberg, Josefin Segelman, Urban Ersson, Mattias Prytz, Eva Angenete, Rebecka Bergström, Markus Mayrhofer, Bengt Glimelius, and Johan Lindberg
More from Amol Akhmade on OncoDaily.
