Miloš Grujić, Head of Department of the subspecialist cabinets at the Center for Radiation Oncology at the University Clinical Center Kragujevac, on LinkedIn:
“New Patient-Reported Outcomes from TALAPRO-2
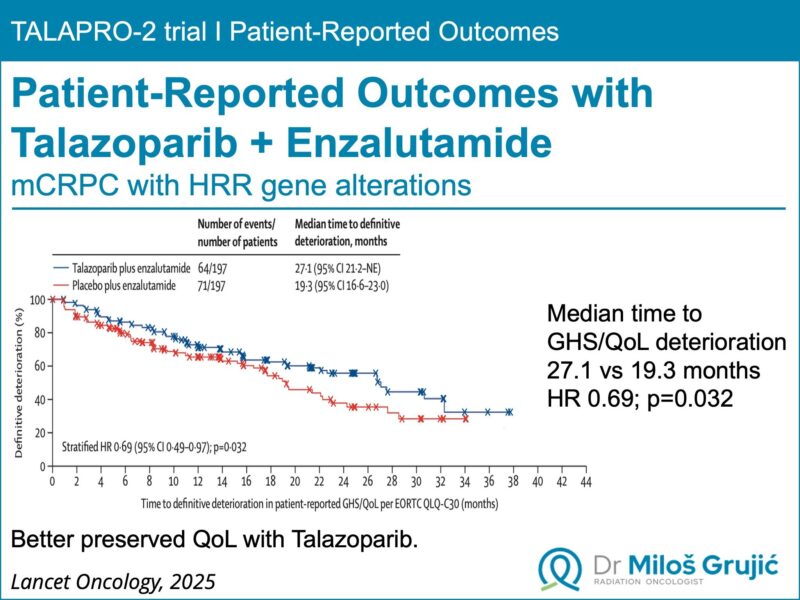
In the latest results published in The Lancet Oncology, talazoparib + enzalutamide demonstrated a significant delay in quality of life deterioration for men with HRR-deficient metastatic castration-resistant prostate cancer (mCRPC).
Median time to GHS/QoL deterioration:
- 27.1 months vs 19.3 months
- HR 0.69 (95% CI: 0.49–0.97), p=0.032
This supports the dual aim of treatment:
Prolonging survival while preserving quality of life.
Incorporating patient-reported outcomes is critical in our evolving standards for mCRPC care.
The study also suggested improved outcomes in urinary and pain symptom control, reinforcing the clinical relevance of durable disease control with PARP + ARPI combinations.
Authors: Andre P Fay et al.

More posts featuring Miloš Grujić on OncoDaily.
