Hagop Kantarjian, Associate Vice President for Global Academic Programs at MD Anderson Cancer Center, shared a recent article he and his colleagues co-authored on X, adding:
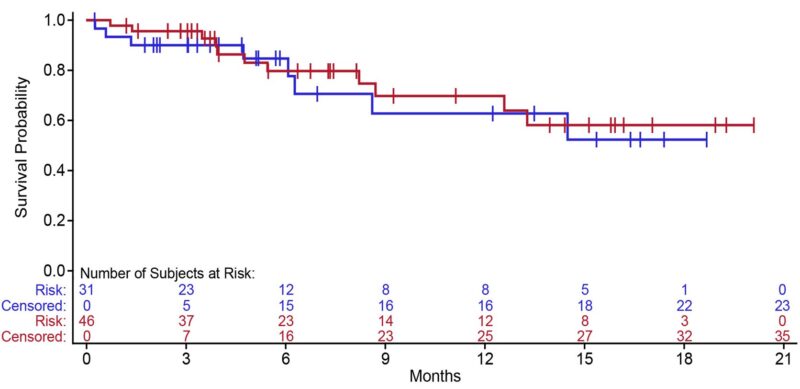
“SQ blinatumomab is highly effective in R-R ALL with CR/CRh rate of 77%, MRD-negativity rate of 91%, and 12-month OS rate of 63-70%.
The 250 µg/500 µg dose was selected as the recommended phase 2 dose.”
Title: Subcutaneous blinatumomab in adults with relapsed or refractory B-cell acute lymphoblastic leukaemia: post-hoc safety and activity analysis from a multicentre, single-arm, phase 1/2 trial
Authors: Elias Jabbour, Federico Lussana, Pilar Martínez-Sánchez, Anna Torrent, José J Rifón, Vaibhav Agrawal, Mar Tormo, Ryan D Cassaday, Thomas Cluzeau, Françoise Huguet, Cristina Papayannidis, Jesús M Hernández-Rivas, Anita Rijneveld, Shaun Fleming, Vladan Vucinic, Boris Böll, Takayuki Ikezoe, Maher Abdul-Hay, Mary L Savoie, Andre C Schuh, Celine Berthon, Stefan Schwartz, Sabina Chiaretti, Junichiro Yuda, Takuya Miyazaki, José González-Campos, Yuqi Chen, Hansen Wong, Jessica Choudhry, Gerhard Zugmaier, Erin Guest, Paul Gordon, Hagop Kantarjian
Read the Full Article on The Lancet Haematology

More posts featuring Hagop Kantarjian.
