Rishabh Jain, Medical Oncologist at AIIMS, shared a post on X about a paper by P.F. Conte et al. published in Annals of Oncology:
“A-BRAVE lesson: Even a ‘negative’ trial can bravely shift the conversation
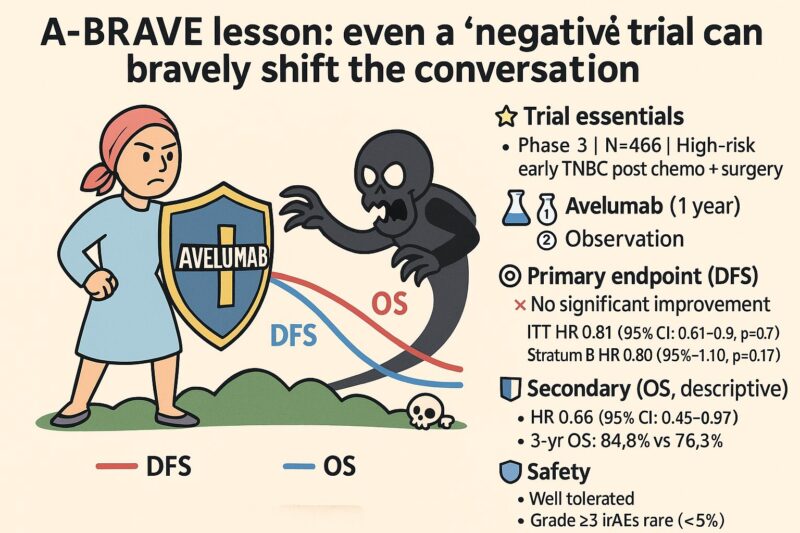
Trial essentials
Phase 3 | N=466 | High-risk early TNBC post chemo + surgery
Arms:
- Avelumab (1 year)
- Observation
Primary endpoint (DFS, co-primary ITT, and Stratum B):
No significant improvement
- ITT HR 0.81 (95% CI: 0.61–1.09, p=0.17)
- Stratum B HR 0.80 (95% CI: 0.58–1.10, p=0.17)
Secondary endpoint (OS, descriptive):
- HR 0.66 (95% CI: 0.45–0.97)
- 3-yr OS: 84.8% vs 76.3%
PD-L1: Prognostic, not predictive
Safety:
- Well tolerated
- Grade ≥3 irAEs rare (<5%)
- Most common: thyroid dysfunction (all G1–2)
Take-home:
Formally negative for DFS, but OS and DDFS show hypothesis-generating signals.
Future trials (e.g., SWOG S1418) will clarify adjuvant ICI’s role.”
Title: A-BRAVE trial: a Phase 3 randomized trial with anti-PD-L1 avelumab in high-risk triple-negative early breast cancer patients
Authors: P.F. Conte, M.V. Dieci, G. Bisagni, P. Schmid, A. Zambelli, F. Piacentini, M. De Laurentiis, A.G. Favaretto, S. Tamberi, G.V. Bianchi, C. Zamagni, S. Cinieri, D.C. Corsi, L. Del Mastro, A. Ferro, A. Gennari, M. Mion, A. Musolino, L. Nicolé, P. Del Bianco, G.L. De Salvo, V. Guarneri
You can read the Full Article in Annals of Oncology.

More posts featuring Rishabh Jain.
