My OncoKB people: Zenocutuzumab has rec’d Breakthrough Designation (BTD) from US FDA for NRG1+ NSCLC.
Looking fwd to it getting tumor agnostic approval based on Alison Schram work showing 42% ORR in PDAC (& 35% in NSCLC).
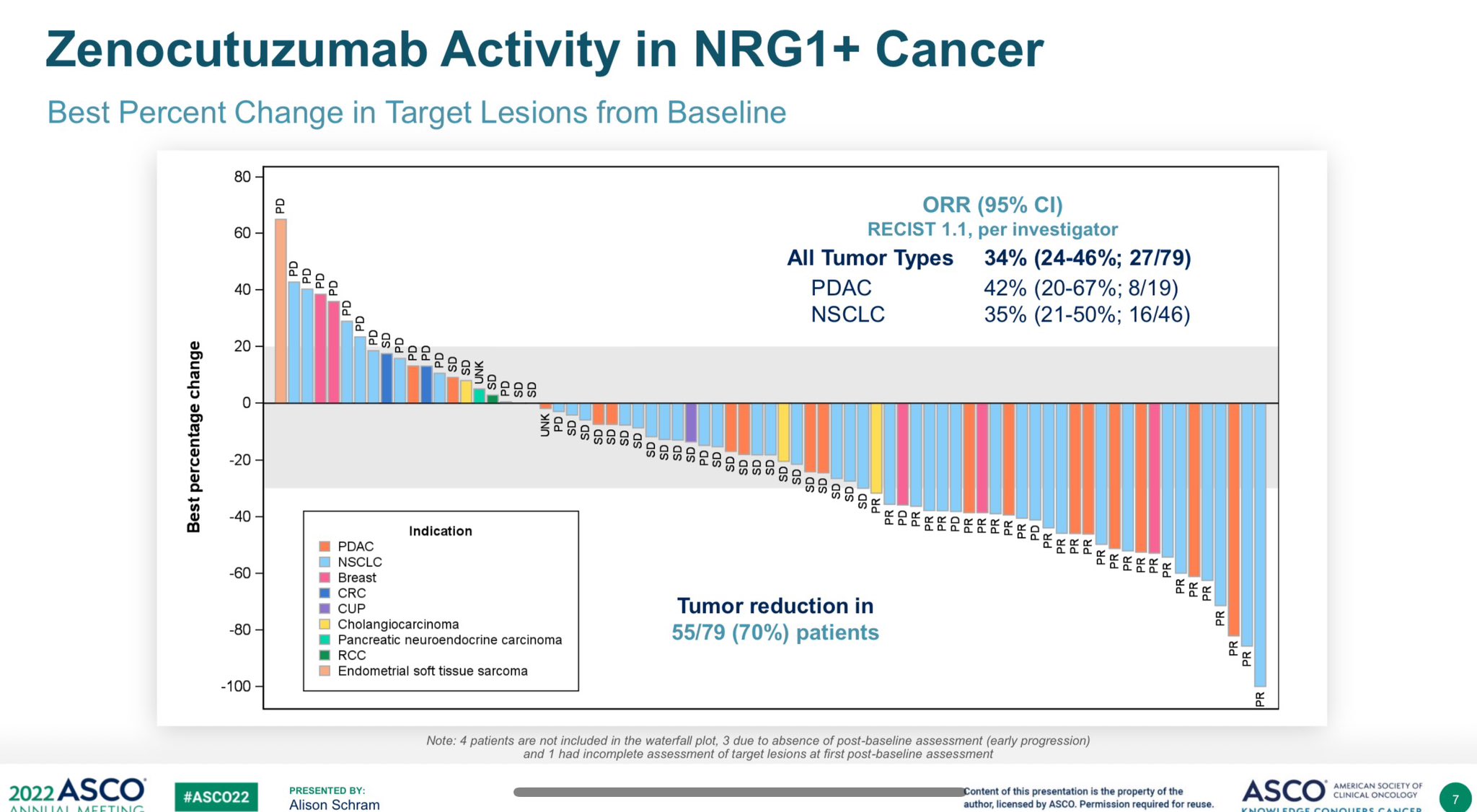
My OncoKB people: Zenocutuzumab has rec’d Breakthrough Designation (BTD) from US FDA for NRG1+ NSCLC.
Looking fwd to it getting tumor agnostic approval based on Alison Schram work showing 42% ORR in PDAC (& 35% in NSCLC).