Esophageal cancer is among the more aggressive cancers of the digestive tract, with many cases diagnosed at an advanced stage. Historically, treatment options were limited, and outcomes were often poor. In recent years, however, immunotherapy has begun to transform the landscape. By helping the immune system recognize and attack cancer cells, these therapies offer new hope—especially for patients with advanced or recurrent disease. But how effective are they? What is the real-world success rate of immunotherapy for esophageal cancer? This article explains what patients need to know based on current clinical trial evidence and regulatory approvals.
Read About Esophageal Cancer on OncoDaily
How Immunotherapy Works in Esophageal Cancer
Immunotherapy for esophageal cancer primarily involves immune checkpoint inhibitors. These drugs block proteins like PD-1 and PD-L1, which tumors use to hide from immune cells. When these checkpoints are blocked, the immune system is reactivated and can begin to attack the cancer.
The most studied agents include nivolumab (Opdivo) and pembrolizumab (Keytruda). These drugs are approved in specific settings depending on tumor type, PD-L1 expression, and prior treatments.
Results from Clinical Trials
One of the pivotal studies that changed treatment guidelines was the KEYNOTE-181 trial, which compared pembrolizumab to standard chemotherapy in patients with advanced esophageal cancer. In those whose tumors had high levels of PD-L1 expression, the results favored immunotherapy: patients lived longer on average and had a better quality of life. Although only a portion of patients experienced tumor shrinkage, those who did often maintained a response for many months or longer.
Similarly, the ATTRACTION-3 trial evaluated nivolumab in patients with esophageal squamous cell carcinoma who had already received chemotherapy. This trial showed a significant survival benefit, even in patients with low or no PD-L1 expression. While not all patients responded, those who did often experienced long-lasting effects.
More recently, immunotherapy has moved into the first-line treatment setting. In the CheckMate 648 study, patients received nivolumab along with either chemotherapy or another checkpoint inhibitor (ipilimumab). Compared to chemotherapy alone, the addition of immunotherapy led to longer survival times, especially in patients with squamous cell histology. A similar study, KEYNOTE-590, looked at pembrolizumab plus chemotherapy in patients with either squamous cell carcinoma or adenocarcinoma. The combination led to improved survival and response rates, particularly in those whose tumors expressed PD-L1 at high levels.
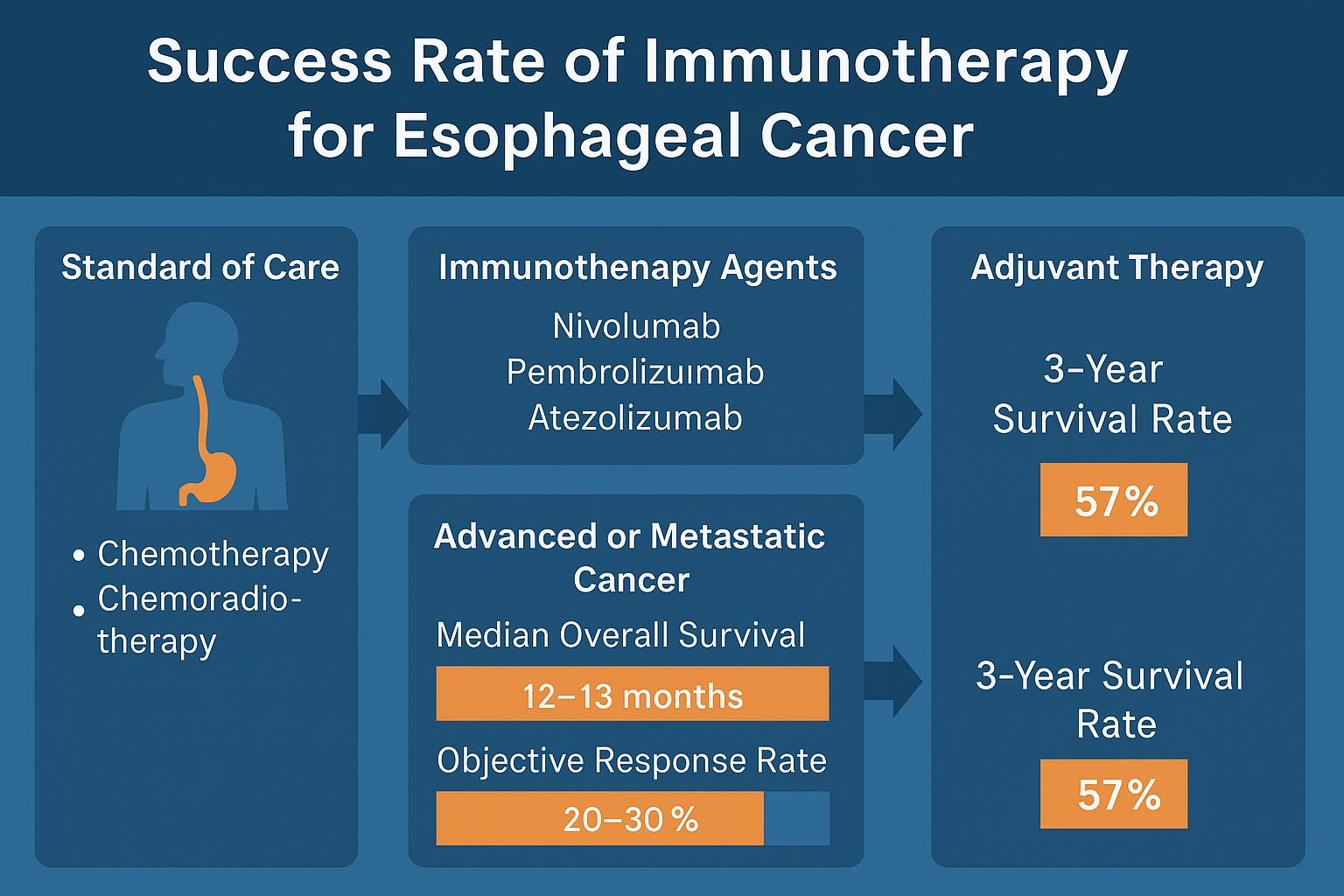
Even patients with early-stage disease may benefit. In the CheckMate 577 trial, patients who had undergone chemoradiation and surgery but still had residual tumor received nivolumab as adjuvant therapy. These patients stayed cancer-free for much longer than those who received placebo, effectively doubling their disease-free survival time.
Success Rate and Durability of Response
Success with immunotherapy in esophageal cancer is not guaranteed for all patients. However, for those who respond, the results can be meaningful and long-lasting. In second-line settings, where chemotherapy options are limited, immunotherapy has extended survival from a median of about six to ten months, with a small percentage of patients living multiple years beyond diagnosis.
In the first-line setting, adding immunotherapy to chemotherapy has improved response rates significantly. For example, more than half of patients receiving nivolumab plus chemotherapy in the CheckMate 648 trial had measurable tumor shrinkage, compared to fewer than one-third on chemotherapy alone. Some patients remain in remission beyond three years, which was rarely seen before the immunotherapy era.
What makes immunotherapy especially promising is not just the average numbers, but the potential for a “tail effect” on survival curves—meaning a subset of patients may live substantially longer than expected, possibly with long-term control of their disease.
What Influences the Effectiveness of Immunotherapy?
Several factors impact how well immunotherapy works in esophageal cancer. Tumors with high PD-L1 expression tend to respond better to pembrolizumab, although nivolumab has shown benefit regardless of PD-L1 status in some trials. The type of cancer also matters—squamous cell carcinoma, which is more common in certain regions like East Asia, appears to be more responsive to immunotherapy than adenocarcinoma.
Whether immunotherapy is used early or late in the course of disease makes a difference. The best outcomes are generally seen when it is used as part of the first-line regimen or as adjuvant therapy after surgery. In later lines of treatment, while the chances of a strong response decrease, the durability of response for those who benefit remains impressive.
Are There Side Effects?
Immunotherapy is typically better tolerated than chemotherapy, but it is not without risk. Some patients develop immune-related side effects, which occur when the immune system begins attacking healthy tissues. Common complications include inflammation of the colon (colitis), liver (hepatitis), lungs (pneumonitis), or hormonal glands (thyroid or adrenal). These side effects are usually manageable with steroids or other medications but require close medical monitoring.
Still, many patients find that the quality of life on immunotherapy is better than on traditional chemotherapy, particularly in the absence of severe toxicity.
What Patients Should Know
The introduction of immunotherapy has changed the outlook for many patients with esophageal cancer. While not every patient will benefit, those who do can experience substantial improvement in survival and a reduction in symptoms. Biomarker testing—particularly for PD-L1—is important in deciding whether immunotherapy is likely to help.
Patients with advanced esophageal cancer should discuss with their oncologist whether immunotherapy is appropriate based on their tumor type, treatment history, and test results. In earlier-stage disease, immunotherapy may be offered after surgery to reduce the risk of recurrence.
For those who are not eligible for approved regimens, participation in clinical trials may provide access to the latest immunotherapy strategies.
Conclusion
Immunotherapy has brought new hope to patients with esophageal cancer, especially for those with squamous cell histology and high PD-L1 expression. Clinical trials have shown that it can improve survival and lead to durable remissions in a meaningful percentage of patients. While success rates vary, the ability of immunotherapy to produce long-lasting responses—even after standard treatments have failed—makes it a valuable tool in the fight against this disease.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
