Pumitamig (BNT327) is an investigational PD-L1–directed bispecific antibody designed to expand the reach of immunotherapy in breast cancer. Triple-negative breast cancer (TNBC), one of the most aggressive subtypes, is defined by the absence of estrogen receptor (ER), progesterone receptor (PR), and HER2 expression. Patients with advanced or metastatic TNBC often face limited therapeutic options, especially those with PD-L1–negative tumors who remain ineligible for immune checkpoint inhibitor–based regimens. Chemotherapy continues to serve as the mainstay of treatment, yet clinical outcomes are poor and durable responses remain rare.
The ROSETTA Breast-01 trial was launched to address this unmet need. This Phase III, randomized, double-blind study evaluates pumitamig in combination with physician’s choice chemotherapy versus placebo plus chemotherapy. The goal is to determine whether pumitamig can improve survival and disease control in PD-L1–negative TNBC, a population historically excluded from immuno-oncology, potentially establishing a new treatment paradigm.
What is Pumitamig (BNT327)?
Pumitamig (BNT327) is an investigational bispecific antibody co-developed by BioNTech and Bristol-Myers Squibb. It is designed to target both PD-L1 and VEGF-A, combining immune checkpoint blockade with anti-angiogenic activity. By simultaneously restoring T-cell function and normalizing tumor vasculature, it aims to enhance immune response and drug delivery within the tumor microenvironment.
Early-phase trials in triple-negative breast cancer (TNBC) and small cell lung cancer (SCLC) have shown encouraging response rates and disease control, including activity in PD-L1–negative tumors where standard immunotherapies fail. Safety has been manageable, though it carries risks related to both VEGF inhibition and immune-related toxicities.
Study Design
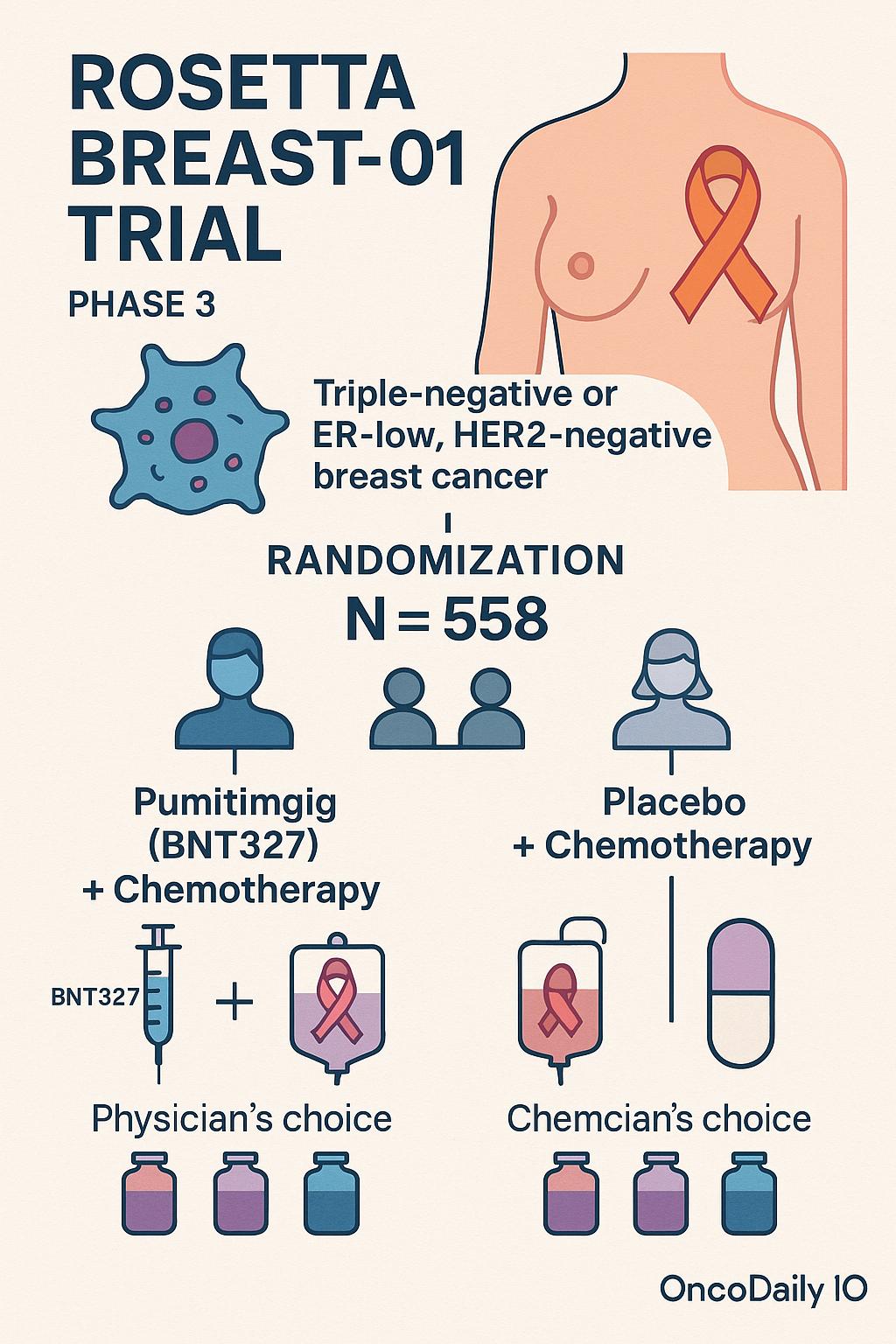
The ROSETTA Breast-01 trial (NCT07173751) is a Phase III, multisite, randomized, double-blind, placebo-controlled study sponsored by BioNTech SE in collaboration with Bristol-Myers Squibb. It is designed for patients with previously untreated, locally recurrent inoperable or metastatic triple-negative breast cancer (TNBC) or ER-low, HER2-negative breast cancer who are ineligible for PD-(L)1 immunotherapy due to PD-L1–negative disease.
A total of 558 participants will be randomized 1:1 to receive either pumitamig (BNT327) plus chemotherapy of physician’s choice or placebo plus chemotherapy. Eligible chemotherapy regimens include paclitaxel/nab-paclitaxel, gemcitabine plus carboplatin, or eribulin. Randomization will be stratified by prior immunotherapy, chemotherapy backbone, geography (East Asia vs. rest of world), and PD-L1 CPS score. Treatment will continue until progression, unacceptable toxicity, or withdrawal, with safety and survival follow-up extending up to 57 months.
Methods and Endpoints
Co-primary endpoints
- Progression-Free Survival (PFS): assessed by blinded independent central review (BICR)
- Overall Survival (OS): time from randomization to death from any cause
Secondary endpoints
Investigator-assessed PFS
- Objective Response Rate (ORR): percentage of patients with complete or partial response
- Duration of Response (DOR): time from first response to progression or death
- Disease Control Rate (DCR): complete response + partial response + stable disease
- PFS and OS rates at 6, 12, 18, and 24 months
Safety assessments will include treatment-emergent adverse events (TEAEs) graded by CTCAE v5.0, dose modifications, and treatment discontinuations. Patient-reported outcomes will be collected using EORTC QLQ-C30, QLQ-BR42, and FACT-G measures to evaluate quality of life, treatment burden, and symptom changes over time.
Expected Results
As a newly initiated Phase III study (estimated start October 2025, primary completion December 2029), results are not yet available. However, pumitamig, a PD-L1–directed bispecific antibody (also known as BNT327, PM8002, BMS-986545), has shown preclinical promise in engaging the immune system through dual targeting.
The trial seeks to determine whether adding this bispecific agent to chemotherapy can overcome the lack of efficacy seen with traditional immunotherapy in PD-L1–negative TNBC, a population historically excluded from immunotherapy-based regimens.
Key Takeaways from ROSETTA Breast-01
- One of the first large-scale trials targeting PD-L1–negative TNBC, addressing a critical unmet need
- Tests pumitamig + chemotherapy versus placebo + chemotherapy
- Employs a rigorous randomized, double-blind design with comprehensive endpoints
- Evaluates survival, safety, and patient-reported quality-of-life outcomes
- Results expected to provide pivotal data for frontline management
- If successful, could expand immuno-oncology use in TNBC beyond PD-L1–positive disease
- Has the potential to reshape standard of care for this difficult-to-treat patient group
