Pediatric soft tissue sarcomas were the focus of a lecture delivered by Dr. Shushan Hovsepyan during the CUHK Sarcoma Masterclass 2025, where she presented recent advances with particular emphasis on rhabdomyosarcoma (RMS) and non-rhabdomyosarcoma soft tissue sarcomas (NRSTS). She highlighted the unique challenges of pediatric sarcoma care, where progress has been slower compared to the adult oncology field, and underlined the importance of centralization of care, clinical trial participation, and international collaboration.

Background: Pediatric Sarcomas in Context
Soft tissue sarcomas represent a rare but highly heterogeneous group of mesenchymal malignancies in children, accounting for about 4–5% of pediatric cancers. Their biology and clinical behavior vary widely, ranging from relatively indolent tumors to highly aggressive malignancies. The spectrum of histologic subtypes also differs by age: rhabdomyosarcoma predominates in childhood, while synovial sarcoma is more frequent in adolescents and young adults, and liposarcoma or leiomyosarcoma are typically adult tumors.
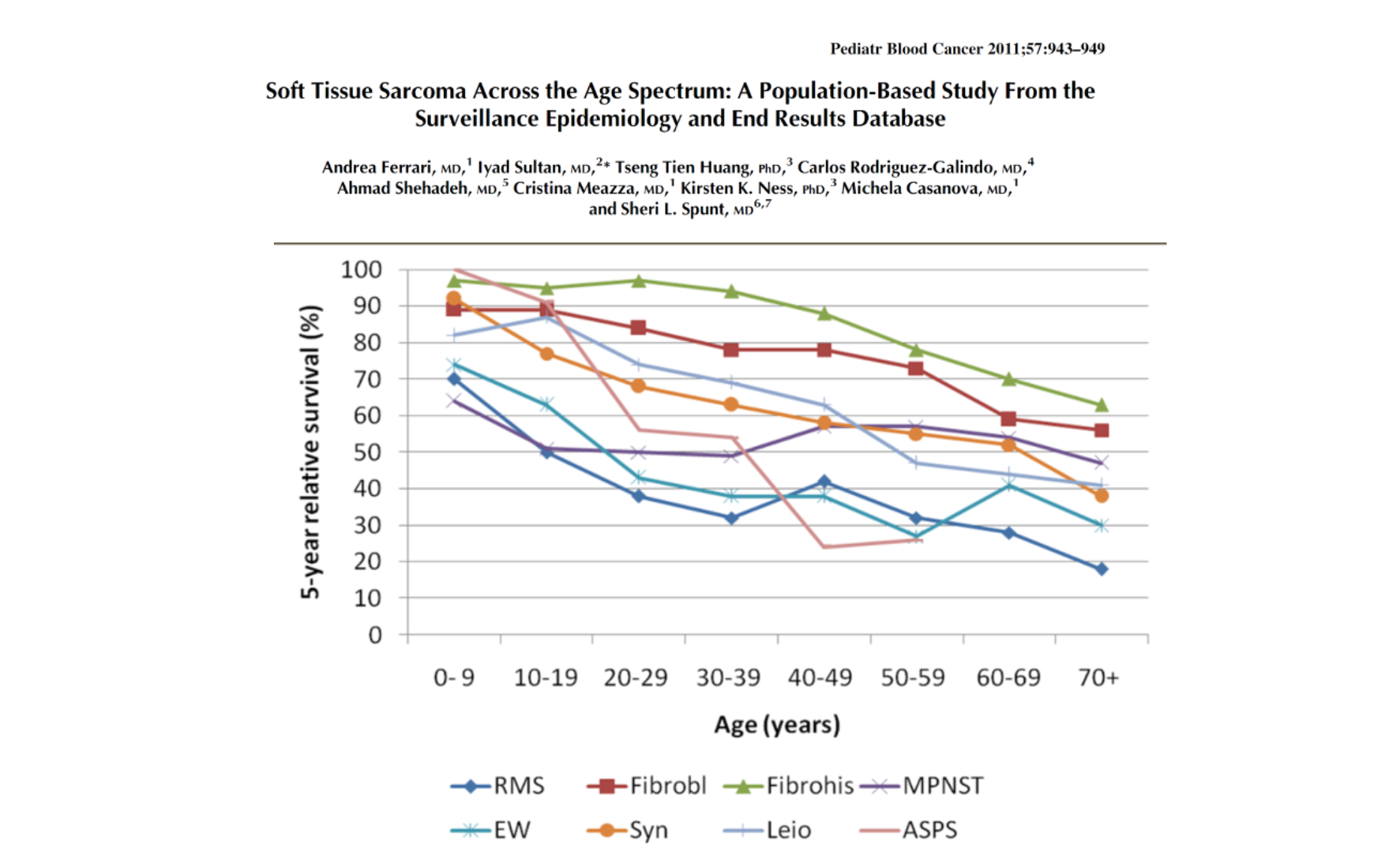
This distribution underscores why pediatric and adult oncologists often adopt different perspectives and strategies when managing STSs. Importantly, outcomes are generally more favorable in pediatric patients than in adults, with survival declining as age increases.
Rhabdomyosarcoma: Risk Stratification and Treatment Advances
Rhabdomyosarcoma is a high-grade malignancy with local invasiveness and a strong tendency to metastasize. Dr. Hovsepyan emphasized that RMS encompasses a wide clinical spectrum—from curable fusion-negative paratesticular tumors in young children to aggressive, fusion-positive cases in adolescents with systemic involvement and poor outcomes.
Modern RMS management rests on three principles: centralization of care in expert centers, high participation in cooperative multi-institutional trials, and risk-adapted treatment strategies. Prognostic factors are used to tailor therapy intensity, with the aim of improving cure rates in high-risk patients while reducing overtreatment and long-term toxicity in favorable cases.
One of the most important advances came from the European EpSSG trial, which tested maintenance therapy after standard induction. The addition of vinorelbine and low-dose cyclophosphamide as maintenance significantly improved survival, raising five-year overall survival from 73.7% to 86.5%. This represented the first randomized trial in over three decades to demonstrate a survival benefit from a new chemotherapy strategy in RMS, and the regimen has since become a new standard of care in Europe and beyond.
Despite this breakthrough, challenges remain. The optimal role of vinorelbine is still being studied, with ongoing trials exploring whether its benefit lies in maintenance or extended therapy duration. Metastatic and relapsed RMS continues to carry a dismal prognosis, and attempts to improve outcomes with agents such as bevacizumab or mTOR inhibitors have been disappointing. Some progress has been made with vincristine–irinotecan–temozolomide combinations, which are now used as relapse standards.
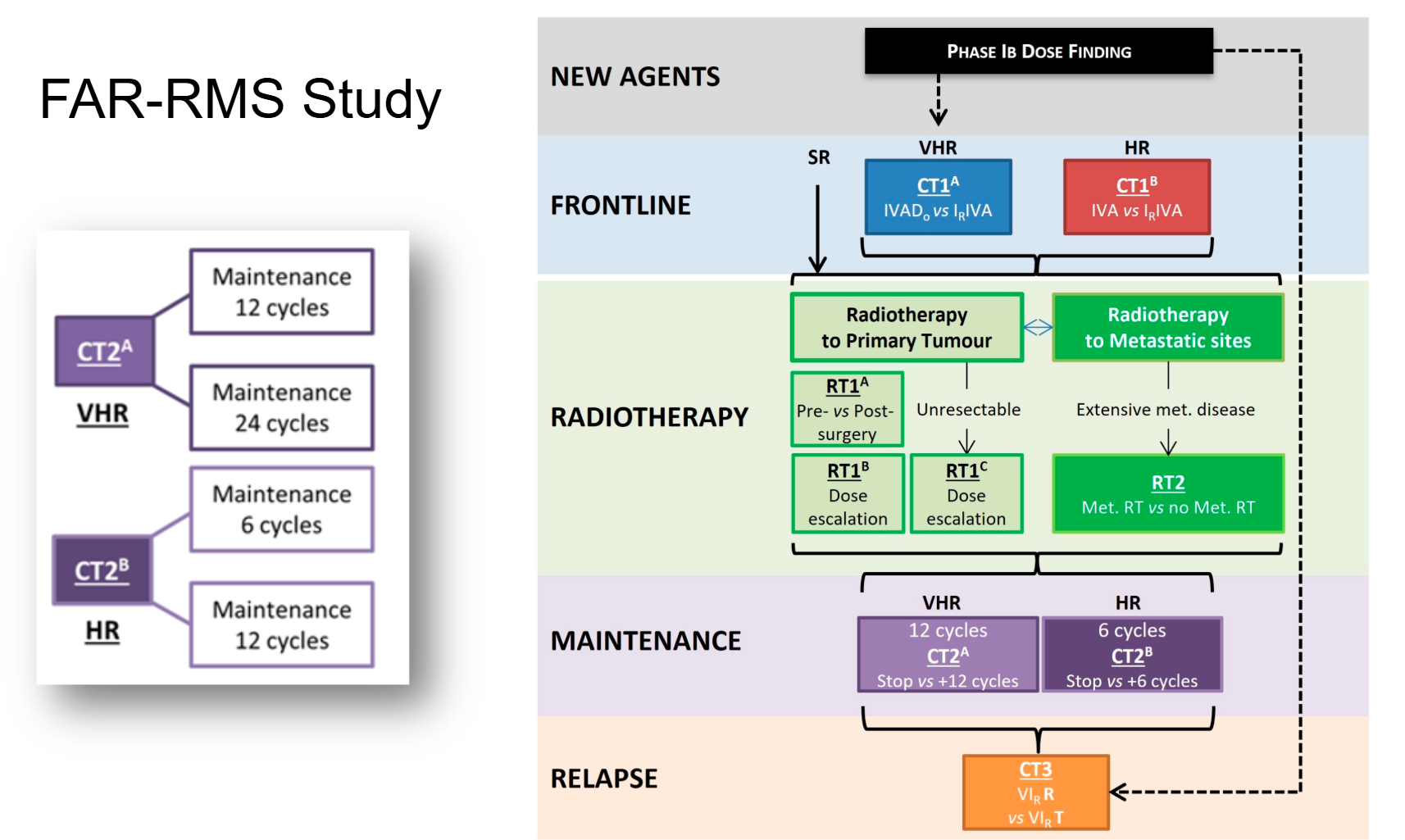
The FAR-RMS Trial and Future Directions
Dr. Hovsepyan described the ongoing FaR-RMS study, a large, multi-arm, multi-stage European trial designed for both children and adults with newly diagnosed or relapsed RMS. Open since 2019, this trial seeks to refine induction chemotherapy combinations, optimize radiotherapy timing and dose, evaluate maintenance strategies, and test new regimens for relapsed disease. With no upper age limit, FaR-RMS represents a paradigm of integrated pediatric–adult collaboration in sarcoma research.
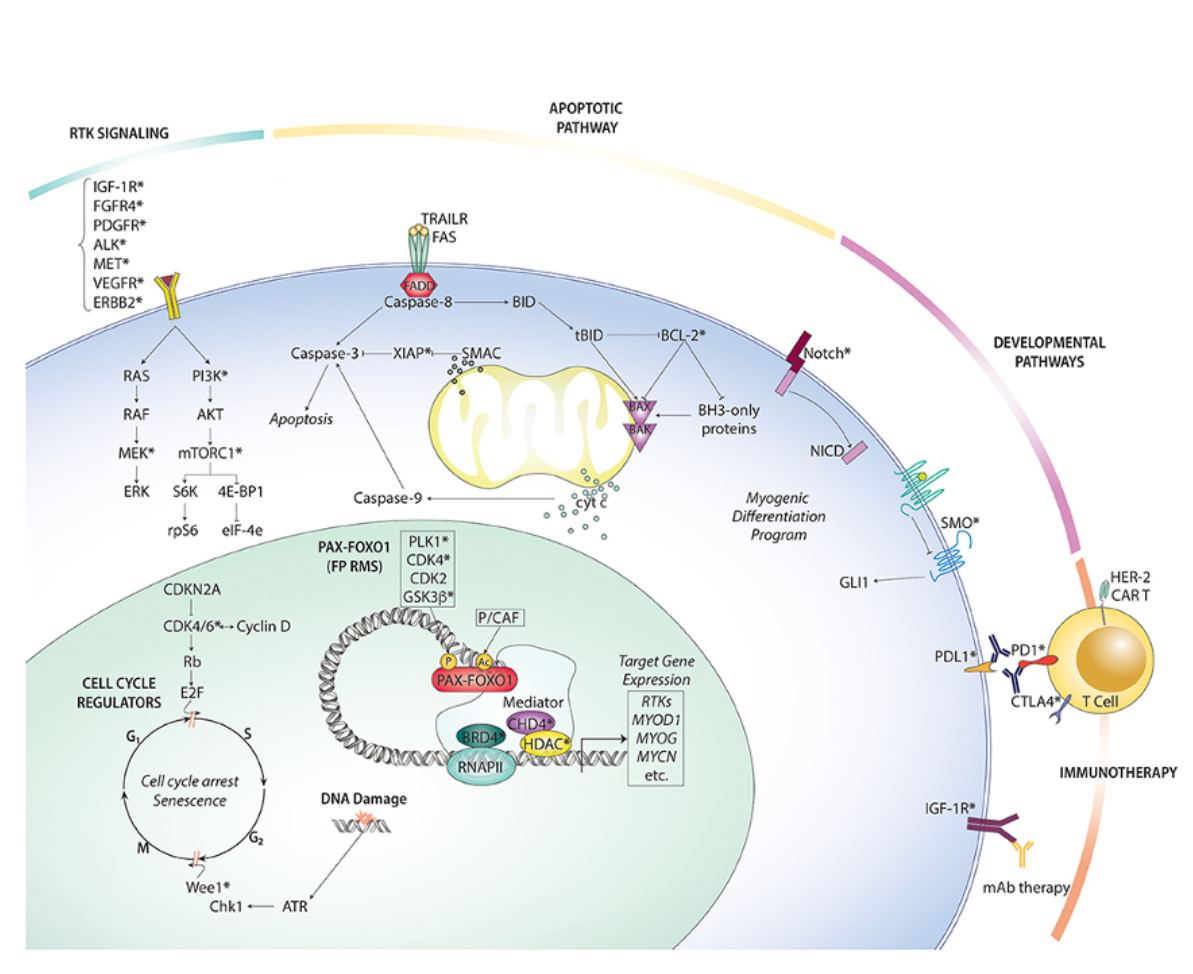
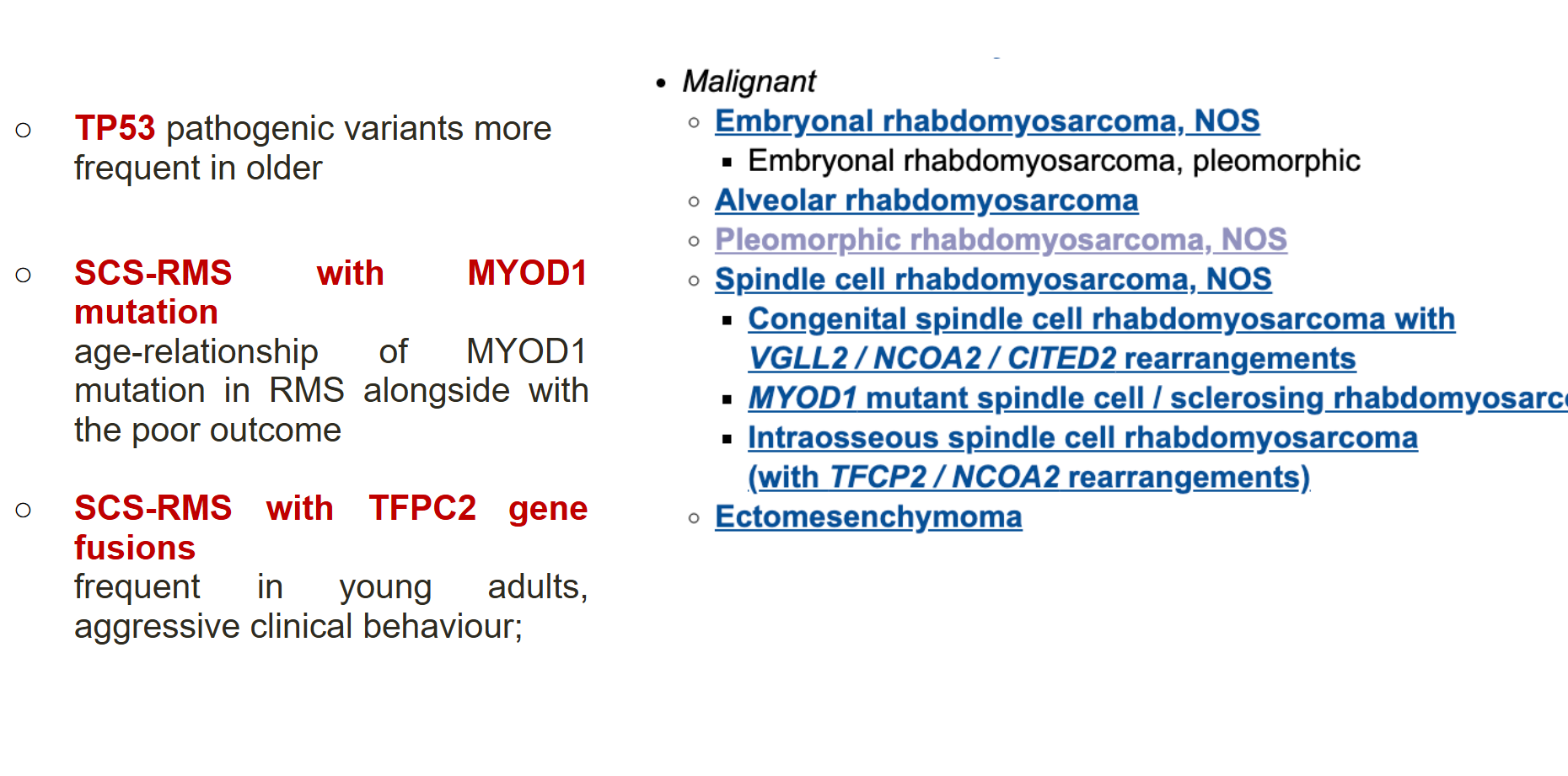
Biology-driven therapy is also a key frontier. RMS subgroups defined by molecular alterations—such as PAX3–FOXO1 fusion, MYOD1 mutations, or TFCP2 fusions—carry distinct prognoses and potential vulnerabilities. Targeted therapies against signaling pathways (FGFR, mTOR, MEK), chromatin modifiers (EZH2, HDAC), fusion proteins (PLK1 inhibition for PAX3–FOXO1), and microenvironmental features (angiogenesis and hypoxia) are all under active investigation.
Non-Rhabdomyosarcoma Soft Tissue Sarcomas
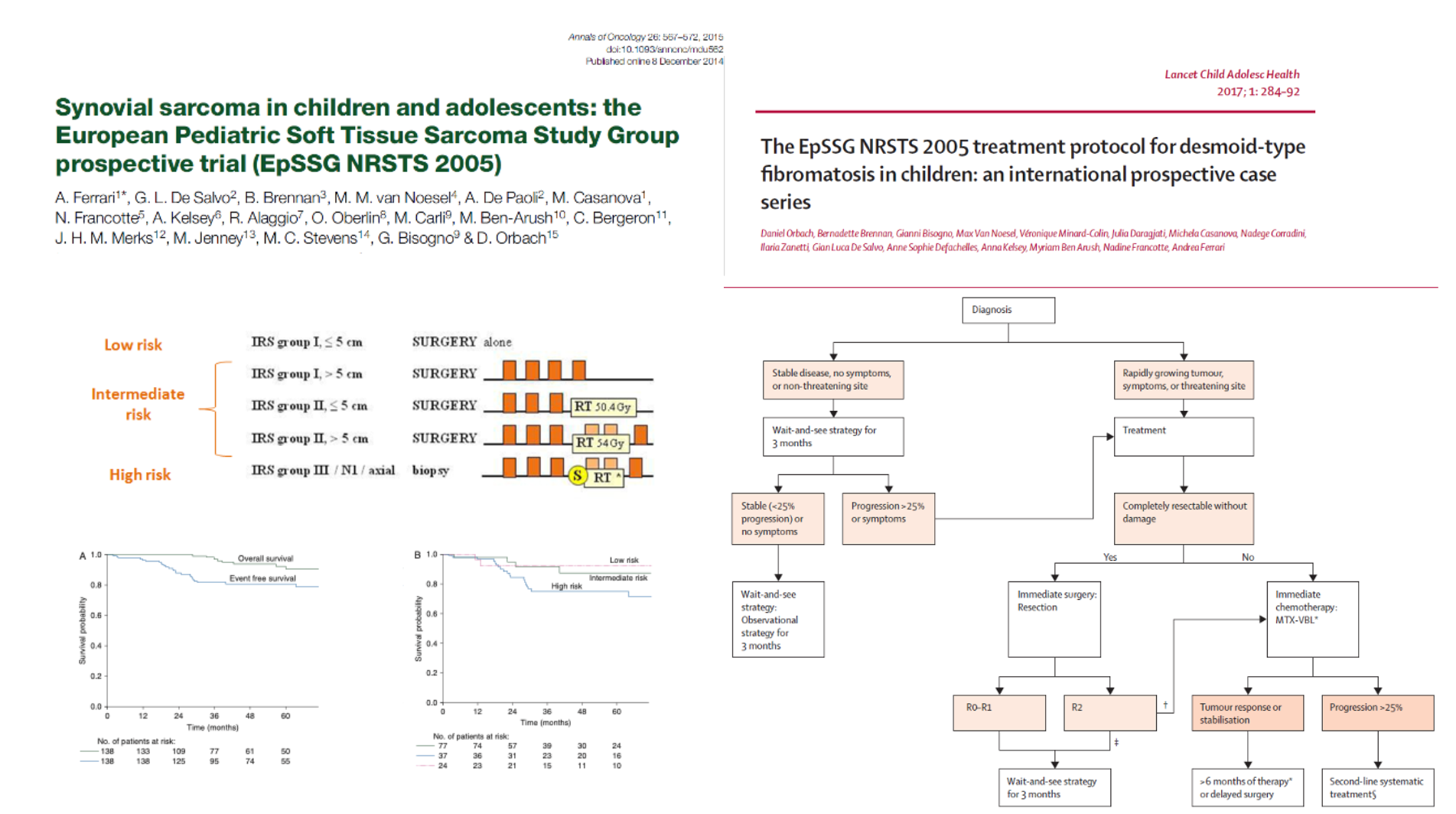
Non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) are rarer, highly heterogeneous, and generally less responsive to chemotherapy. Historically, they were managed with RMS-based protocols, which failed to capture their biological diversity. A major shift occurred in 2005 when both North American and European groups launched dedicated NRSTS trials. The EpSSG NRSTS 2005 study established risk-adapted treatment standards, confirming that adjuvant chemotherapy and radiotherapy can be omitted in low-risk disease while unresected or high-risk tumors benefit from neoadjuvant ifosfamide–doxorubicin regimens.
NRSTS encompasses many distinct histotypes, each with unique biology and outcomes. For example, infantile fibrosarcoma has excellent prognosis and responds dramatically to targeted NTRK inhibitors such as larotrectinib and entrectinib, while desmoplastic small round cell tumor is highly aggressive with poor survival. Synovial sarcoma remains a central focus, particularly in adolescents and young adults, while inflammatory myofibroblastic tumors can be effectively treated with ALK inhibitors.
Age-Related Differences and the AYA Gap
Adolescents and young adults (AYA) with RMS or NRSTS often have worse survival outcomes compared to younger children. This survival gap is likely multifactorial, involving differences in tumor biology, delays in diagnosis, lower enrollment in clinical trials, and suboptimal treatment intensity. Evidence suggests that AYA patients treated with pediatric-style intensive regimens achieve better outcomes, while adults receiving reduced or altered chemotherapy regimens fare poorly. This highlights the need for harmonized, cross-age treatment strategies and stronger integration between pediatric and adult oncology communities.
Targeted Therapies
Dr. Hovsepyan also discussed the emerging role of targeted therapies in pediatric and adolescent soft tissue sarcomas. In synovial sarcoma, immunotherapies targeting cancer-testis antigens such as MAGE-A4 (including agents like afami-cel and leticell) have demonstrated response rates of about 40%, with some durable responses lasting more than a year, though so far these approaches remain confined to adult settings. In desmoid tumors, γ-secretase inhibitors have shown encouraging activity, providing a non-surgical alternative for a disease that often required extensive operations.
For infantile fibrosarcoma and other NTRK-fusion–positive tumors, NTRK inhibitors such as larotrectinib and entrectinib have produced dramatic and rapid tumor regressions, often allowing safer surgery. Similarly, inflammatory myofibroblastic tumors with ALK rearrangements can now be effectively managed with ALK inhibitors. These advances highlight the potential of integrating novel agents into pediatric sarcoma care, offering new therapeutic avenues beyond conventional chemotherapy.
Conclusion
Dr. Shushan Hovsepyan’s lecture emphasized that pediatric soft tissue sarcomas represent a unique and diverse clinical challenge, distinct from their adult counterparts. Progress in rhabdomyosarcoma has been marked by the adoption of maintenance chemotherapy, the development of international collaborative trials such as FaR-RMS, and the integration of biology into risk stratification and therapeutic targeting.
For non-rhabdomyosarcoma subtypes, risk-adapted approaches and histotype-specific strategies have begun to replace older, one-size-fits-all regimens. Across both RMS and NRSTS, the central themes remain clear: the importance of specialized care, inclusion in cooperative trials, and international collaboration to pool resources for rare diseases. As the field moves forward, the integration of molecular insights with optimized standard therapies holds the promise of narrowing the survival gap for adolescents and adults, and of delivering more precise, personalized treatment for children with soft tissue sarcomas.
You can read the abstract here.
