Sergio Cifuentes, Cancer Research Project Manager at CENEIT México, posted on X:
“Critiques on the PLATFORM study design
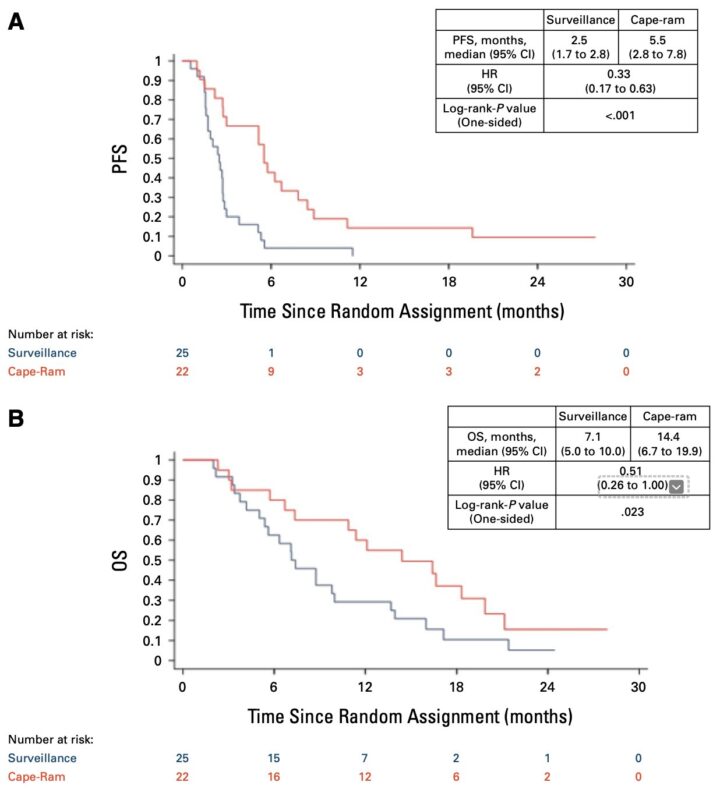
(Cape-Ram maintenance after 1L chemo in advanced esophagogastric adenocarcinoma)1. Phase II, low power
Only 47 patients randomized. Limited statistical power undermines OS analysis and toxicity data robustness. Exploratory, not practice-changing.2. Early arm closure
Sponsor withdrawal led to premature termination of the cape-ram arm, introducing potential selection bias and affecting study validity.3. Suboptimal control: surveillance
Control arm was observation, not active treatment. This may inflate cape-ram efficacy vs. an inactive comparator. Not reflective of clinical practice.
4. Obsolete vs. current standard
By design time, CM649 had already shown benefit of chemo+IO followed by IO maintenance. PLATFORM excluded IO entirely—limits its relevance today.5. Notable toxicity
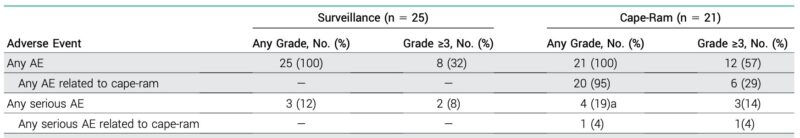
Grade ≥3 AEs occurred in 57% of cape-ram vs. 32% surveillance. The toxicity burden is significant, especially for patients with controlled disease post-induction.
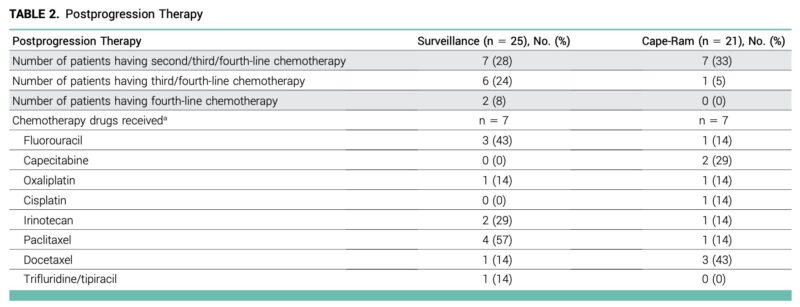
6. Lack of post-progression standard
Despite early closure, most patients did not receive standard second-line chemo-IO. This questions OS benefit attribution solely to cape-ram.”
Title: Maintenance Capecitabine Plus Ramucirumab After First-Line Chemotherapy in Patients With Advanced Esophagogastric Adenocarcinoma: Results From the Randomized PLATFORM Study
Journal: JCO Oncology Advances
Authors: Anderley Gordon, David Cunningham, Zayn Rajan, Caroline Fong, Clare Peckitt, Laura Satchwell, Susan Cromarty, Ian Chau
More posts featuring Sergio Cifuentes on OncoDaily.