Raffaele Colombo, Associate Director of Medicinal Chemistry at Zymeworks, shared a post on X by Stephen V Liu, adding:
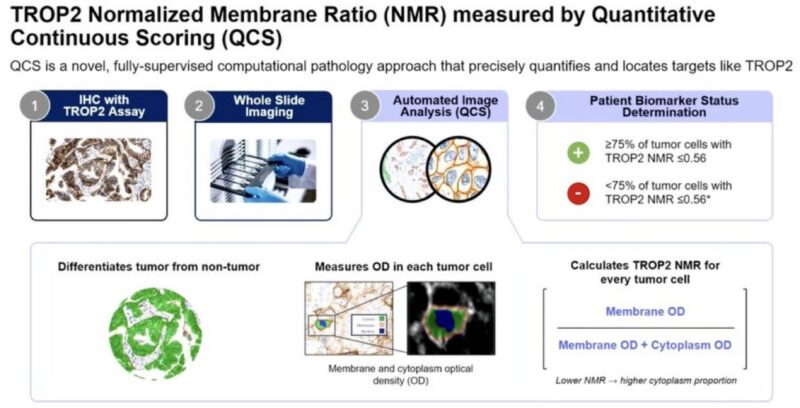
“Based on AstraZeneca’s QCS for TROP2,
Roche Diagnostics received FDA Breakthrough Device Designation (BDD) for the VENTANA TROP2 (EPR20043) RxDx assay!Nice spotlight presentation at AACR25.”
Read more about Roche’s FDA Breakthrough Device Designation for AI-driven NSCLC diagnostic here.
Quoting Stephen V Liu‘s post:
“Dr. Marina Garassino at WCLC24 Presidential Plenary presents Normalized Membrane Ratio of TROP2 as a biomarker for datopotamab deruxtecan in TROPION-Lung01 (Dato-DXd vs docetaxel in previously treated NSCLC which previously showed PFS benefit with Dato-DXd.“

Learn more about Raffaele Colombo and TROPION-Lung01 on OncoDaily.
