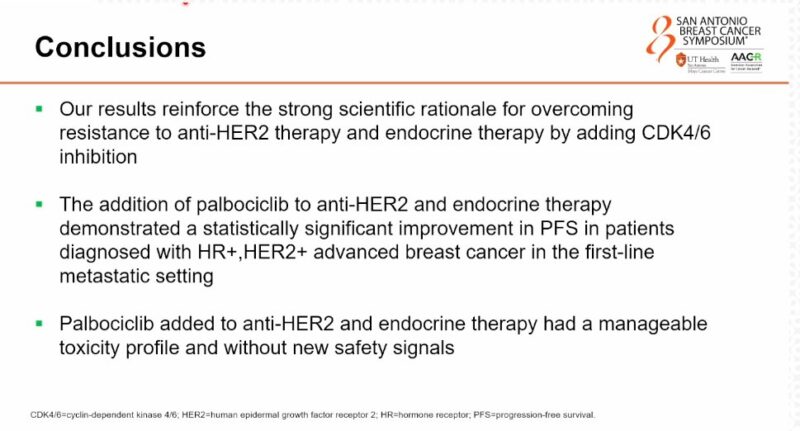
Pre-clinical data showed that CDK 4/6 inhibition plays a role in HER2+ disease. Phase 2 trials suggest synergistic antitumor activity and potential efficacy of palbociclib when combined with anti-HER2 therapy, especially in HR+/HER2+ breast cancer.
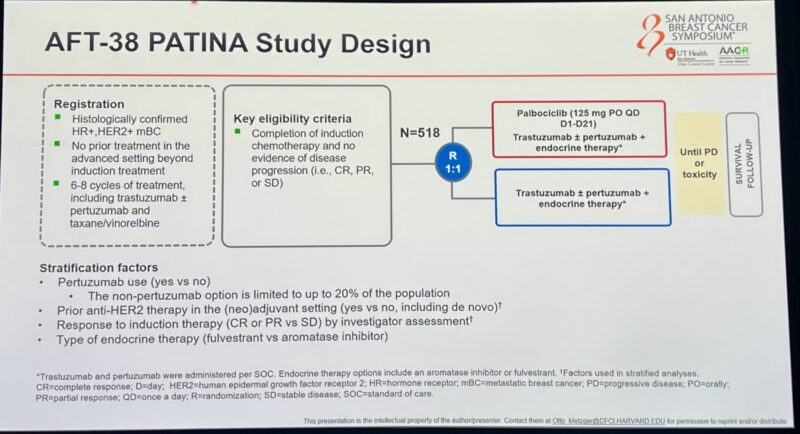
The aim of PATINA is to evaluate the efficacy and safety of adding palbociclib to anti-HER2 therapy and endocrine therapy maintenance after induction treatment in the first-line setting for HR+/HER2+ metastatic breast cancer. The study was published in 2018 at ESMO Annals of Oncology and results were presented at SABCS24.
Healthcare professionals shared their insights about PATINA study on social media:
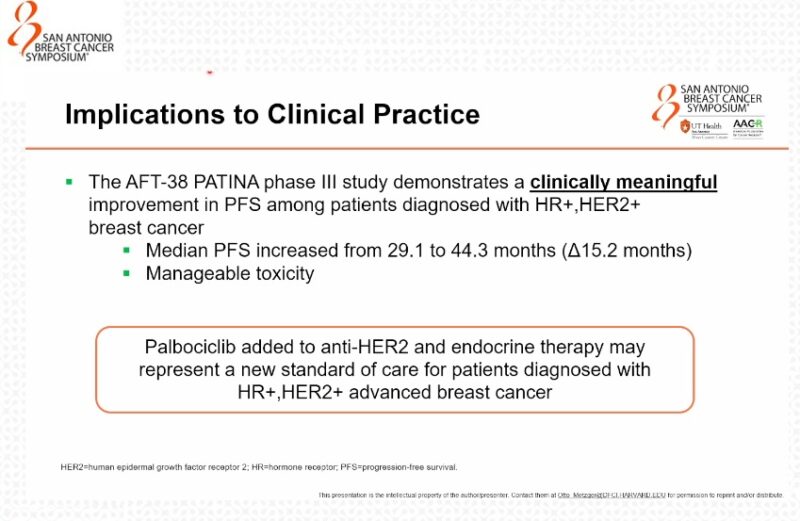
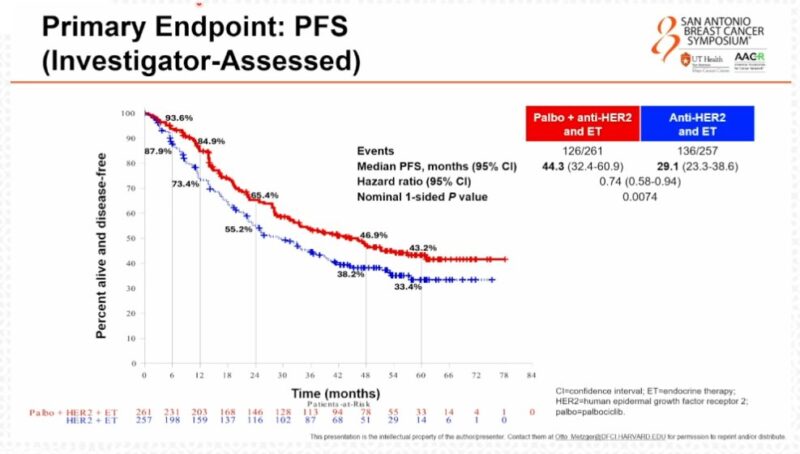
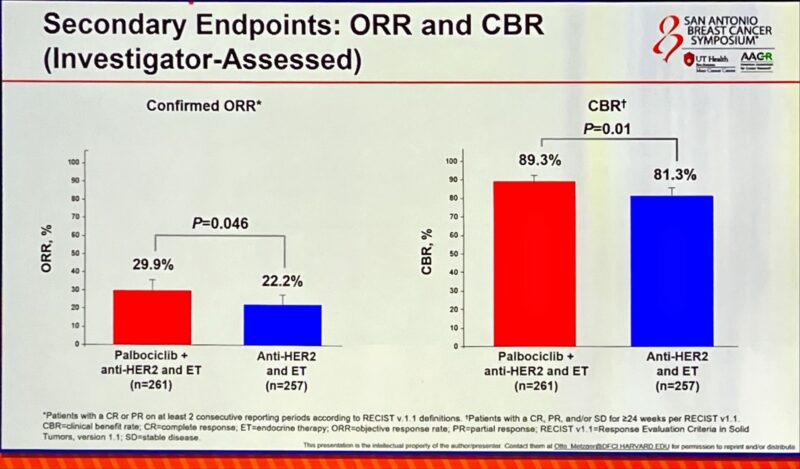
“PATINA—the addition of palbociclib to HP after discontinuation of docetaxel in 1st line mHER2+BC—results in an improvement in median PFS from 29.1 months to 44.3 months, a HR 0.74.”
“PATINA
Patients have gotten through a tough chemotherapy, if you add something toxic to their subsequent therapy, it should increase OS!
We need to stop saying toxicities are ‘manageable’, the palbociclib group had much more toxicities, it should be said like that, without spin.”
“Unprecedented outcomes in 1st line HER2+ Breast Cancer! It is amazing to see this improvement for ER+ HER2+ Breast Cancer! Practice changing data!”
Dana-Farber’s Breast Oncology Center:
“Dr. Otto Metzger presented recent data on the PATINA Study.
A Randomized, Open Label, Phase III Trial to Evaluate the Efficacy and Safety of Palbociclib + Anti-HER2 Therapy + Endocrine Therapy vs. Anti-HER2 Therapy + Endocrine Therapy after Induction Treatment for HR+/HER-Positive.”
“PATINA study of induction THP > maintenance HP + ET +/- palbociclib in ER+HER2+ MBC.
Takeaway: after 6 cycles induction TH, patients can expect nearly 4 years of maintenance HP/ET/palbo on average before re-needing chemo/ADC!”
“PATINA trial
Palbociclib is making a comeback.
A practice-changing study in triple-positive breast cancer.”
“Otto Metzger presents the practice-changing results from the PATINA phase 3 trial: adding palbociclib to maintenance ET after 1 line THP for HR+/HER2+ MBC significantly and meaningfully improved PFS (44 vs 29 months, HR 0.74, p=0.007).
44 months!”
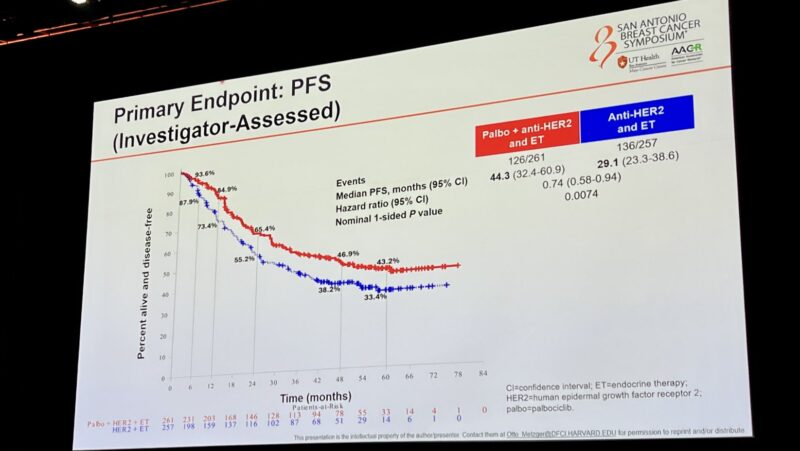
“Practice changing findings from PATINA presented by Otto Metzger.
- n=518 HR+/HER2+ mBC
- all received THP followed by HP+ET +/- palbociclib
- PFS was 29 vs 44 months with palbociclib
- Toxicity consistent with prior studies”
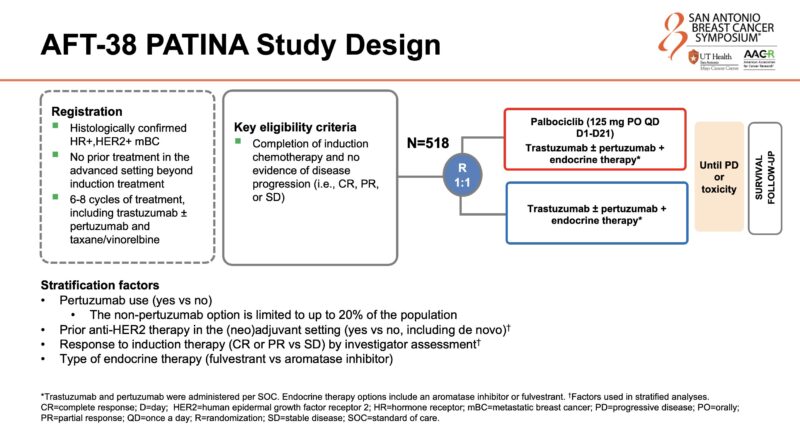
“PATINA significant and clinically important results. Collaboration between researchers. Congrats!”

“Amazingly presented by Otto Metzger at SABCS24:
PATINA Phase III trial testing Palbociclib + Anti-HER2 Therapy + ET vs. Anti-HER2 Therapy + ET in 1st-line HER2+ breast cancer maintenance.
- PFS: 29.1 vs. 44.3 months with Palbo (52-month follow-up).
- OS: Immature.”
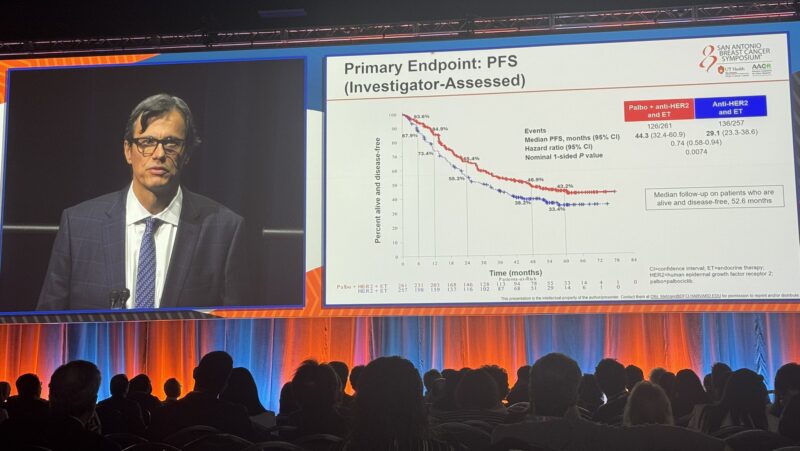
“The AFT-38 PATINA phase III study demonstrates a clinically meaningful improvement of Palbociclib added to anti-HER2 and endocrine therapy in PFS among patients diagnosed with HR+/HER2+ breast cancer. How will this stand when we see the results of DB-09?”
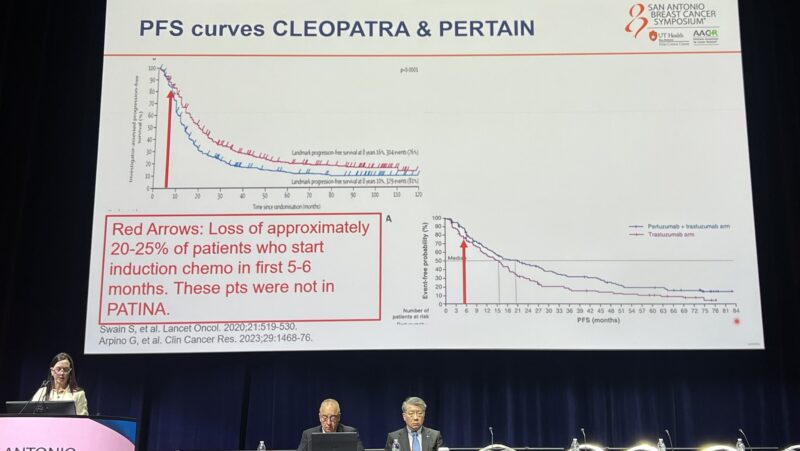
“Making a critical point about how/why PATINA had such a high performance control arm, Dr. Sara Hurvitz describes how the recruitment window excluded patients who had a brisk progression, changing the shapes of the curves.”
Read the post “PATINA trial: Phase III Trial of Palbociclib, Anti-HER2 Therapy, and Endocrine Therapy vs. Anti-HER2 Therapy and Endocrine Therapy in HR+/HER2+ Metastatic Breast Cancer” on oncodaily.com.
