Nicola Ferrari, Director of Translational Science Lead for Respiratory and Immunology at AstraZeneca, shares a post on LinkedIn:
“Fifty years of monoclonals: the past, present and future of antibody therapeutics
This Review focuses on the 50-year odyssey from the advent of hybridoma technology in 1975 to the present era of antibody therapeutics and their clinical application to targeting B cells and HER2+ cancers.
- In 1975, Köhler and Milstein invented hybridoma technology for the generation of murine monoclonal antibodies with predetermined antigen-binding specificity.
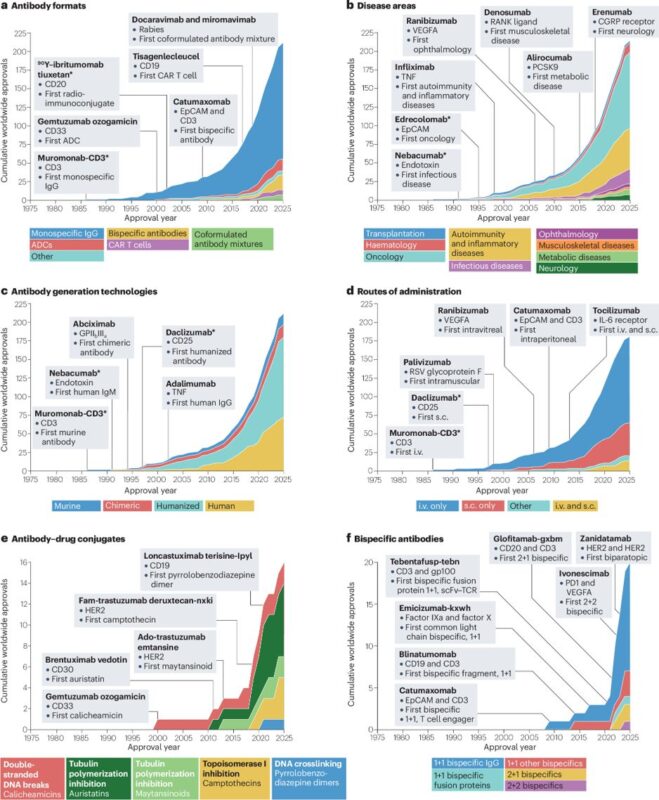
- The transformative impact of hybridoma technology over the past 50 years is hard to overstate. Indeed, hybridoma provided a foundation that has led to at least 212 approved antibody therapeutics to date that have benefited millions of patients.
- Most antibody therapeutics are approved for indications in oncology (48%), or in autoimmunity and inflammatory diseases (25%). Almost all current antibody therapeutics are approved for delivery either by i.v. infusion (~63%), s.c. injection (~25%) or both i.v. and s.c. (8%).
- Antibody therapeutics have been dominated from the outset by monospecific IgG, which represent ~74% of currently approved antibodies.
- Beyond IgG, antibody therapeutics have blossomed into multiple alternative formats, including bispecific antibodies and antibody–drug conjugates. Additionally, antibody fragments have been developed as stand-alone therapeutics and to target cell therapies, notably chimeric antigen receptor T cells.”
Title: Fifty years of monoclonals: the past, present and future of antibody therapeutics
Journal: Nature Reviews Immunology
Authors: Andrew C. Chan, Greg D. Martyn, Paul J. Carter

More posts featuring antibody therapies on OncoDaily.
