Amol Akhade, Consultant Medical Oncologist at Suyog Cancer Clinics, shared his post on X:
“And it got USFDA approval today. It is better strategy than post op nivo alone as probably neo adjuvant IO does the job .
Will I like to read full paper published? Yes. FDA is really fast now a days.”

Quoting Amol Akhade‘s shared post:
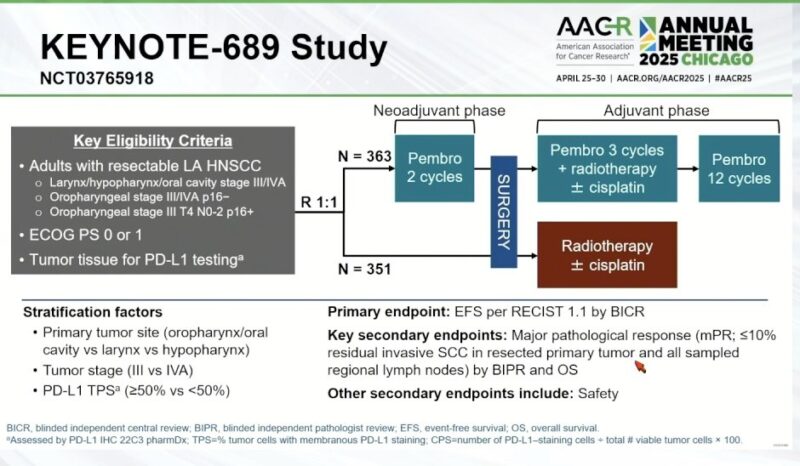
“KEYNOTE-689 Update at AACR25
Neoadjuvant + adjuvant pembrolizumab in resectable LA-HNSCC
EFS HR: 0.73 (95% CI 0.58–0.92)
Median EFS: 51.8 mo vs 30.4 mo
CPS ≥10 subgroup: HR 0.66 (95% CI 0.49–0.88)
2-yr EFS: 75% vs 62%
Early & sustained KM curve separation
Benefit seen across PD-L1 strata and tumor stages
Hypopharynx subgroup showed no clear benefit (tiny n). OS still awaited. New perioperative IO standard incoming?”