Piotr Wysocki, Professor of Medicine and Head of the Department of Oncology at Jagiellonian University Hospital, shared on LinkedIn:
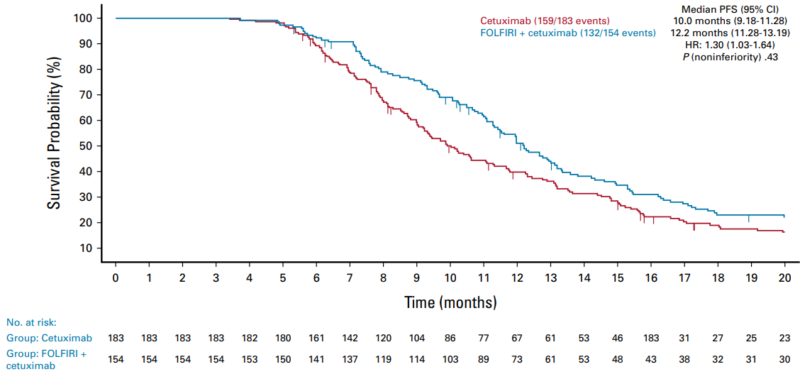
“A phase III open-label study (ERMES) has evaluated the role of single-agent maintenance with cetuximab within first-line treatment in advanced colorectal patients. The study enrolled 606 (RAS/BRAFwt) patients who were randomized in a 1:1 ratio to (A) standard chemotherapy (FOLFIRI+cet – 5-fluorouracil+leucovorin+irinotecan combined with cetuximab) given until progression or to (B) eight cycles of FOLFIRI+cet followed by maintenance with cetuximab alone. The coprimary endpoints were a non-inferior progression-free survival (PFS) in the modified per-protocol (mPP) population (at least 9 cycles of FOLFIRI+cet) and a lower incidence of grade G3-4 adverse events (AEs) for arm B compared with arm A.
With the median follow-up of 22.3 months, the PFS in the mPP was 10.0 and 12.2 months for cet maintenance and continuous FOLFIRI+cet, respectively (P of noninferiority = .43). In the intention-to-treatment (ITT, at least one cycle) the PFS was 9.0 versus 10.7 months (P =.39). The overall survival was 35.7 versus 30.7 months (P = .119) and 31.0 versus 25.2 months (P = .32) in the mPP and ITT population, respectively. Maintenance therapy with cetuximab was associated with a much lower incidence of G3-4 AEs than in the continuous FOLFIRI-cet arm (20.2% v 35.1%).
The ERMES study did not demonstrate noninferiority of maintenance with cetuximab alone, which cannot be assumed as a standard approach in clinical practice. However, it may represent an interesting option in patients demonstrating poor tolerance of the FOLFIRI+cet combination.
These results are not surprising since many earlier studies and cohort analyses suggested that cetuximab monotherapy was insufficiently active as maintenance in early-line settings.
In my daily practice, I often use a de-escalation strategy in patients achieving disease control with FOLFIRI+cet (after 3-4 months), which is based on the cetuximab+irinotecan combination. This combination was evaluated 20 years ago by David Cunningham et al. (NEJM 2004;351:337-345), who demonstrated its significant superiority over cetuximab alone regarding responses and time-to-progression (in patients unselected for RAS, BRAF status). Getting rid of a 46-hour 5-FU infusion and offering the patients biweekly 2-hour visits is very well received by the patients and still allows for much better disease control than cetuximab monotherapy.”
Read further.
Source: Piotr Wysocki/LinkedIn
