Shaalan Beg, Senior Advisor for Clinical Research at the National Cancer Institute, shared a post on X:
“Is 2025 going to be the year Pan-RAS inhibitors make it into the clinic?
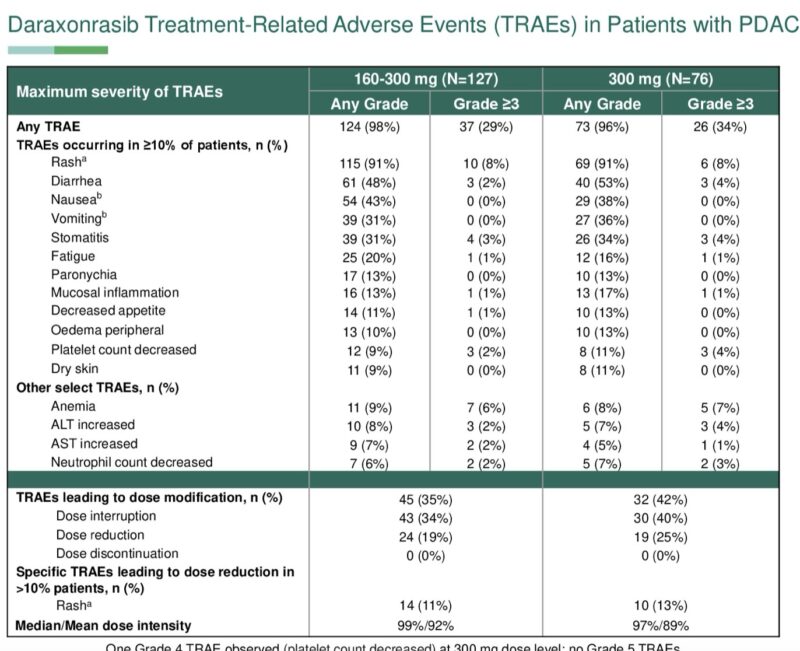
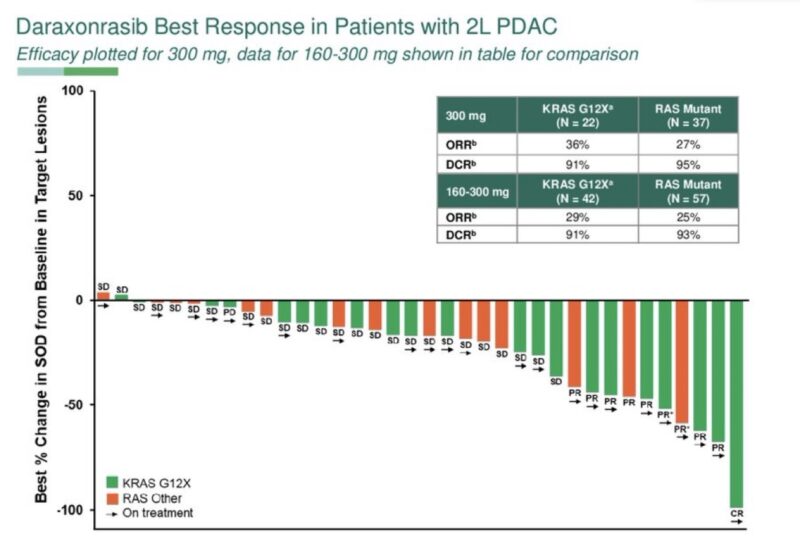
Pan-RAS inhibitor shows early/deep molecular response (and 36% response rate) for G12X mutated pancreatic cancer.
(Significant AEs: rash, diarrhea, nausea, vomiting).”
Pashtoon Kasi, Medical Director of GI Medical Oncology at City of Hope, shared this post on X, adding:
“GI25 finally it has a name.
DARAXonRASib
RMC – 6236 | MULTI (pan-RAS)
Also:
– Elironrasib (RMC-6291 | G12C)
– Zoldonrasib (RMC-9805 | G12D)
We are participating in the registration studies. A lot of enthusiasm about it.”
Read Full article at ASCO Daily News.
