Akhil Santhosh, Medical Oncologist at Dr BR Ambedkar Institute Rotary Cancer Hospital, shared a post on X:
- Small randomised phase 2 trial
- Used durva only in neoadjuvant phase with nab pacli and dose dense EC
Authors: A. Schneeweiss, J. Huober, M. Braun, J. Rey, J.-U. Blohmer, J. Furlanetto, D.-M. Zahm, C. Hanusch, J. Thomalla, C. Jackisch, P. Staib, T. Link, K. Rhiem, C. Solbach, P.A. Fasching, V. Nekljudova, C. Denkert, M. Untch.

- One seperate window cohort where single dose of durva given 2 weeks prior
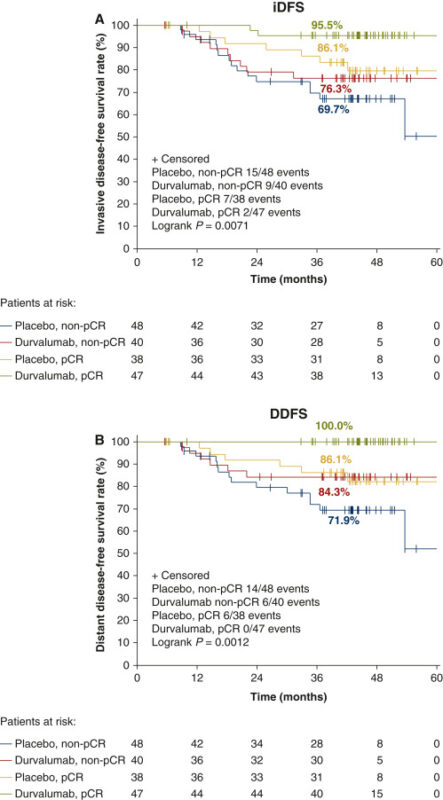
- Primary endpoint point was PCR, study was heavily powered for the same, which was not met(insignificant increase)
- Improved Disease-Free Survival, DDFS and Overall Survival, but all are exploratory analyses with no relevant meaning
- Improvement in path Cr irrespective of PDL1 expression, Residual Stromal TILS prognostic for improved IDFS .”