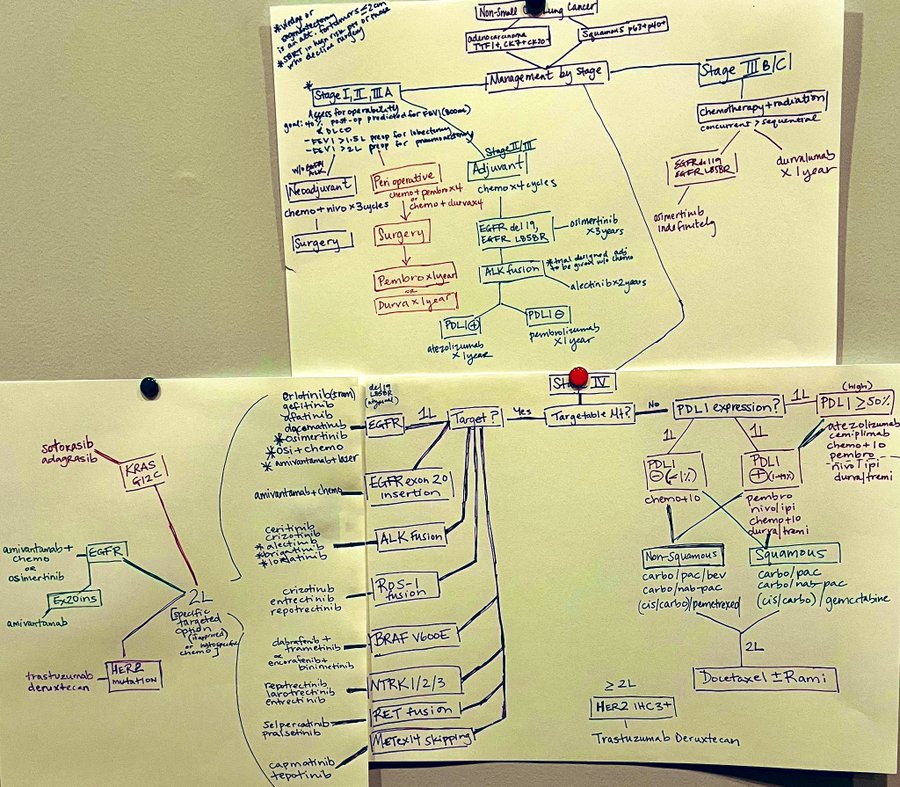
Jennifer Marks, Thoracic Oncologist at Georgetown Lombardi, shared a post on X, referencing an earlier post from July 31st 2024:
“An updated version of the previous one given the new NSCLC FDA Oncology approvals: AEGEAN (perioperative durva plus chemo x 4 durva x 1 year) and MARIPOSA (1L ami plus lazer in mEGFR).”
Source: Jennifer Marks/X