FDA oncology shared on X:
“OCE’s Gautam Mehta and colleagues summarize the FDA accelerated approval of fam-trastuzumab deruxtecan-nxki—the first targeted treatment option for patients with HER2-mutated non-small cell lung cancer – via The Oncologist Journal.”
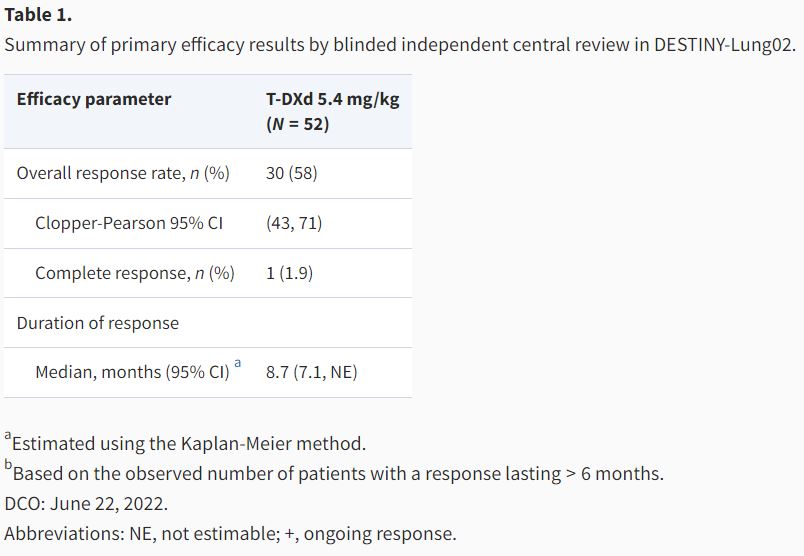
FDA approval summary: fam-trastuzumab deruxtecan-nxki for unresectable or metastatic non-small cell lung cancer with activating HER2 mutations
Autors: Gautam U Mehta, Paz J Vellanki, Yi Ren, Anup K Amatya, Pallavi S Mishra-Kalyani, Lili Pan, Jeanne F Zirkelbach, Yuzhuo Pan, Jiang Liu, Stephanie L Aungst, Claudia P Miller, Mirat Shah, Nam Atiqur Rahman, Marc Theoret, Paul Kluetz, Richard Pazdur, Julia A Beaver, Harpreet Singh
Source: FDA Oncology/X