Zolbetuximab is a monoclonal antibody that targets Claudin 18.2 (CLDN18.2), a tight-junction protein that is overexpressed in certain cancers, particularly gastric (stomach) and gastroesophageal junction (GEJ) adenocarcinomas. On October 18, 2024, the U.S. Food and Drug Administration (FDA) approved Zolbetuximab for the treatment of locally advanced unresectable or metastatic HER2-negative and gastric gastroesophageal junction (GEJ) adenocarcinoma in patients whose tumors express Claudin 18.2 (CLDN18.2). It was approved for use in combination with fluoropyrimidine- and platinum-containing chemotherapy as a first-line treatment.
Which company produced Zolbetuximab?
Zolbetuximab (brand name Vyloy) was developed by Astellas Pharma Inc., a Japanese multinational pharmaceutical company. The company was established on April 1, 2005, following the merger of Yamanouchi Pharmaceutical Co., Ltd., and Fujisawa Pharmaceutical Co., Ltd. Astellas focuses on therapeutic areas such as oncology, urology, immunology, ophthalmology, and women’s health. The company is headquartered in Tokyo, Japan, and is known for developing innovative medicines for unmet medical needs.
How does Zolbetuximab work?
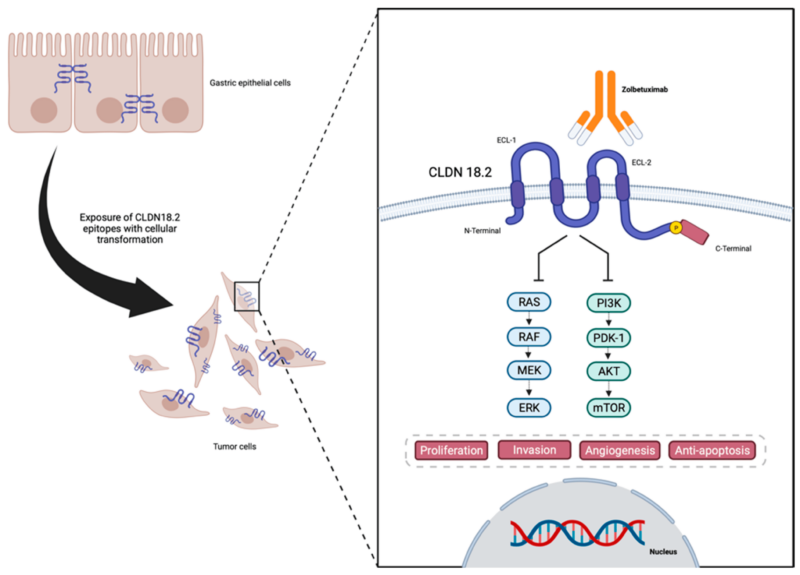
Zolbetuximab is a monoclonal antibody designed to target Claudin 18.2 (CLDN18.2), a protein normally found in the tight junctions of gastric mucosal cells. Claudin 18.2 (CLDN18.2) is a membrane protein found in the lining of the stomach, where it helps maintain the tight junctions between cells. These junctions act like biological glue, keeping cells tightly packed together to form a protective barrier. Under normal conditions, Claudin 18.2 remains hidden within these junctions, shielded from the immune system.
Cecília, M., De Jesus, V. H., Jácome, A., Donadio, M. D., Aruquipa, M. P., Fogacci, J., Cunha, R. G., Da Silva, L. M., & Peixoto, R. D. (2023). Claudin 18.2 as a New Biomarker in Gastric Cancer—What Should We Know? Cancers, 16(3), 679. https://doi.org/10.3390/cancers16030679
However, in certain cancers, such as gastric (stomach) and gastroesophageal junction (GEJ) adenocarcinomas, changes in the structure of tumor cells cause Claudin 18.2 to become abnormally exposed on the cell surface. This exposure occurs because cancer disrupts the normal integrity of tight junctions, allowing Claudin 18.2 to be detected outside the cell.
This unique characteristic makes it an ideal therapeutic target for Zolbetuximab. Once administered, zolbetuximab selectively binds to Claudin 18.2 on tumor cells, marking them for destruction. This triggers the body’s immune system to attack the cancer cells through two key mechanisms:
- Antibody-Dependent Cellular Cytotoxicity (ADCC):
After attaching to the tumor cells, zolbetuximab recruits and activates immune effector cells, such as natural killer (NK) cells and macrophages. These immune cells then attack and destroy the tumor cells, leading to a reduction in tumor burden. - Complement-Dependent Cytotoxicity (CDC):
Zolbetuximab also activates the complement system, a part of the immune response that enhances the ability to clear pathogens and damaged cells. This activation results in the formation of the membrane attack complex (MAC), which punctures the tumor cell membrane, ultimately leading to cell lysis and death.
By leveraging these immune-mediated processes, zolbetuximab helps the body recognize and eliminate cancer cells more effectively. Its selectivity for Claudin 18.2-positive tumors ensures that healthy tissues remain largely unaffected, minimizing unwanted side effects.
What Cancers is Zolbeduximab Approved to Treat?
Zolbetuximab is an innovative treatment designed for patients with advanced gastric (stomach) and gastroesophageal junction (GEJ) adenocarcinoma. The treatment is specifically for HER2-negative tumors that express CLDN18.2, a protein found in certain cancer cells. These cancers can be particularly aggressive and difficult to treat, but zolbetuximab offers a targeted approach that helps the immune system recognize and attack tumor cells. Currently, the FDA has approved zolbetuximab as a first-line treatment for patients with locally advanced, unresectable, or metastatic gastric and GEJ adenocarcinoma. Learn more about stomach cancer, symptoms, and causes on OncoDaily.

