Amol Akhade, Senior Consultant Medical Oncologist and Hemato-Oncologist at Suyog Cancer Clinics and Reliance Hospitals, shared a post on LinkedIn:
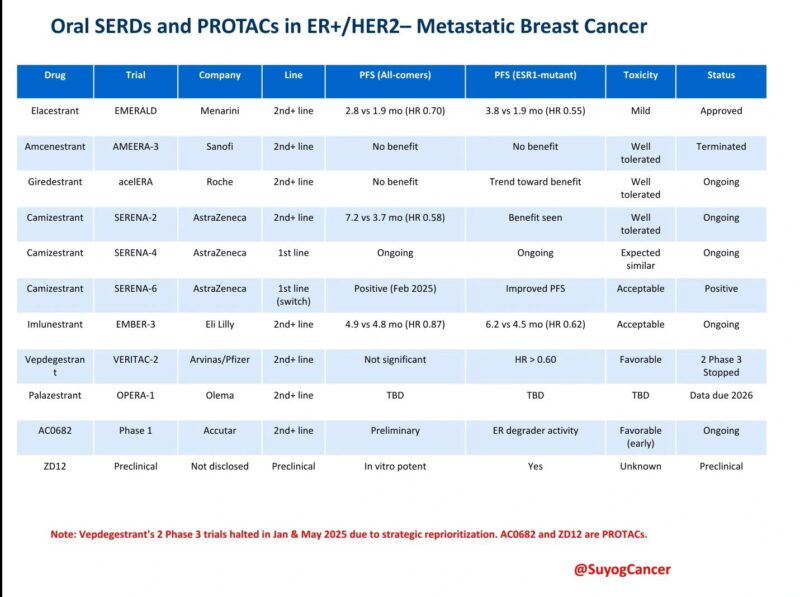
“Oral SERDs and PROTACs in ER+/HER2– Metastatic Breast Cancer: Trial Landscape Update
The therapeutic race is heating up in ER+/HER2– mBC. Here’s a quick snapshot of where key agents stand:
FDA-Approved:
Elacestrant (Orserdu): Approved Jan 2023 for ESR1-mut mBC (EMERALD trial)
In Trials:
Camizestrant (SERENA-6): PFS benefit in early-switch strategy (ESR1+ ctDNA-guided) Imlunestrant (EMBER-3), Giredestrant (acelERA), Palazestrant (OPERA-1): Still ongoing
Withdrawals:
Vepdegestrant (ARV-471): Despite PFS signal in ESR1-mut group, both Phase 3 trials (VERITAC-3 and atirmociclib combo) were withdrawn in Jan and May 2025 by Arvinas/Pfizer as part of pipeline reprioritization
Emerging PROTACs:
AC0682 (Accutar): Now in Phase 1 ZD12: Potent preclinical ERα degrader
One Slide. All Trials. All Statuses.”
Further Reading:
Elacestrant (Orserdu): Uses in Cancer: Side effects: Dosage: Expectations, and More
Elacestrant: What patients need to know?
VERITAC-2 Trial Update: Vepdegestrant Meets Primary Endpoint in ESR1-Mutant Breast Cancer
