Rami Manochakian, Hematology-Oncology Fellowship Director at Mayo Clinic, shared on X/Twitter:
“HOT OFF THE PRESS
U.S. FDA approves Amivantamab WITH CHEMOTHERAPY for 1stLine treatment of advanced non-small cell Lung Cancer with EGFR Exon20 mutations. Approval based on Papillion trial which showed increased mPFS compared with chemo alone: 11.4 vs 6.7 months.”

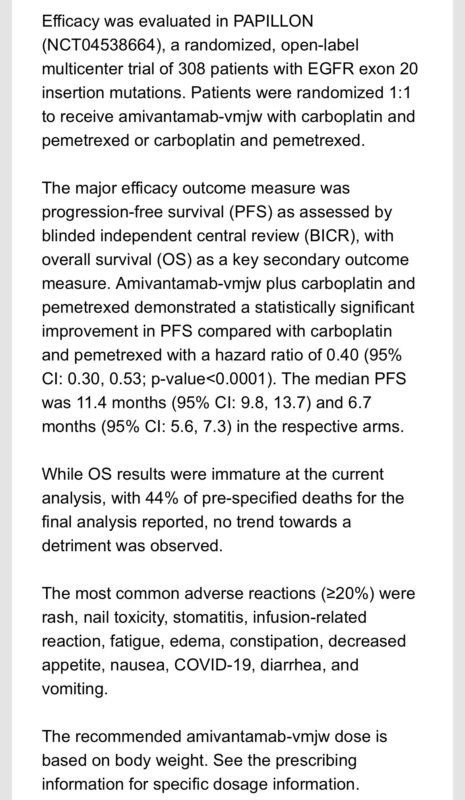
Source: Rami Manochakian/X
