Tian Zhang, Associate Director of Clinical Research at Simmons Comprehensive Cancer Center and Director of Clinical Research at UT Southwestern Medical Center, shared a post on X:
“Best practices for 2L+ treatment Metastatic Urothelial Carcinoma with Petros Grivas and me …assuming EV+P 1L
FGFR3 alts: THOR/erda
Trop-2: sacituzumab govitecan
HER2 IHC 3+: T-DXd
Support by edu grants: Astellas, Gilead Sci, Merck, Seagen CME, Bonum CE
What’s your specialty?
– Medical Oncology
– Surgical Oncology
– Other MD/DO
– APP, RN, PharmD, Other HCP
COI and CME info
1. Answer Pre-survey
2. Review
3. claim CME

mUC SOC changing rapidly
EV+P in 1L
What does it mean for subsequent therapy?
68 years old Female with mUC s/p EV + P, on pembro monotherapy x12m (d/ced EV)
FGFR3-TACC3 fusion + and cisplatin eligible
New liver mets
Chemo-naive cisplatin eligible patients
– Post-1L IO monotherapy
– 2L gem+cisplatin (or ddMVAC) pref’d
– Erda for FGFR3 mut/fusion (THOR level 1 evidence for erda post-IO)
– Cisplatin ineligible patients can get gem+carbo or EV (or erda if FGFR3+ alt)
Let’s consider a patient case…
74 years old Female s/p gem/ + cisplatin
– Avelumab maintenance x12m
– No FGFR3 alterations, HER2 IHC 0
– New liver mets
What would you do next?
– Enfortumab Vedotin
– Erdafitinib
– Nivolumab
– Pembrolizumab
Preferred 2L therapy for cisplatin-ineligible pts after 1L ICI monotherapy
EV or gem + carbo or erdafitinib (if FGFR alt+)
Pembro alone is one of2L therapy options for pts who progress after 1L platinum-based chemo and have not received ICI
Other 2L options(depending on prior therapy):
– Erda for FGFR3 alterations
– EV monotherapy
Taxane if no access to
– Nivo or avelumab if no access to pembro + ICI naïve
– T-DXd for HER2 3+ IHC (gastric Ca scoring)
– Sacituzumab govitecan (FDA indication withdrawn)
Atezolizumab EMA approved as monotherapy
– 1L in cisplatin-ineligible + PD-L1 +ve
– Based on IMvigor 210 and 130 trials
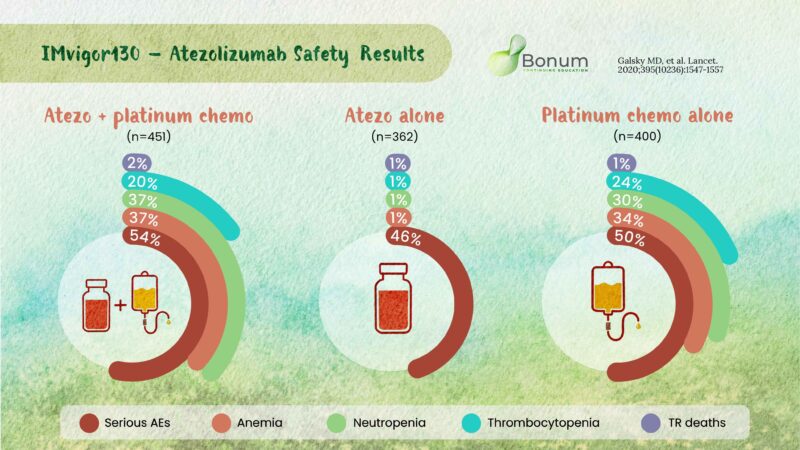
IMvigor 130:
– mPFS significantly longer in atezo+plat/Gem vs plat/Gem alone
– mOS not sign. longer in atezo grps vs plat/Gem alone

IMvigor130 safety results
– Fewer AEs
– withdrawal of any agent in atezo only group
– Most common TRAEs mainly related to chemotherapy:
1. Anemia
2. Neutropenia
3. Thrombocytopenia

Even with pembro 24 mo PFS rate in KEYNOTE-045 was 12.4%
Patients likely to need subsequent line therapy NCCN guidelines
RE: next treatment?
Options (if not given prior):
– EV
– Erda for FGFR3 alt
– T-DXd for HER2 IHC 3+ (gastric Ca scoring)
– Saci(indication withdrawn, still in NCCN guidlines)
Erdafitinib
– Jan 19, 2024 FDA regular approval
– Patients with mUC and FGFR3alt with progression after 1L therapy
– based on THOR1 trial of pts previously treated with PD-1/PD-L1 ICI
– Not for patients with no prior PD-1/PD-L1 if ICI eligible
Erdafitinib cont.
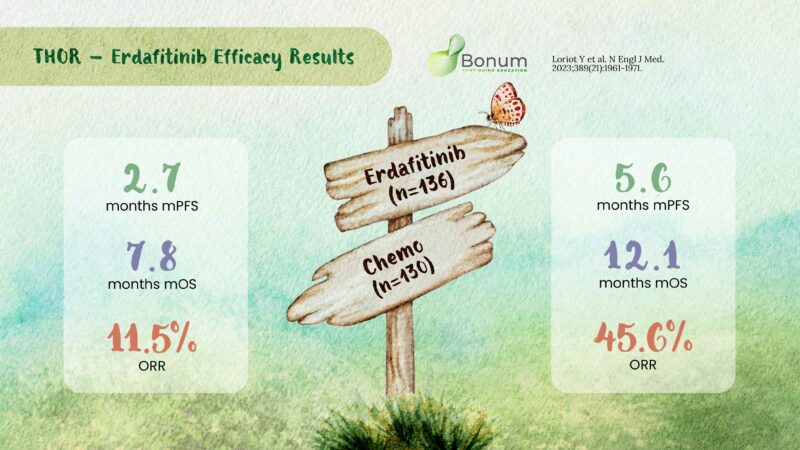
– mOS and mPFS significantly in erdafitinib group vs taxane or vinflunine
– THOR1 trial efficacy results
Erda. Safety
– Grade ≥3 TRAEs occurred in 45.9% with erdafitinib and 46.4% with chemo
– Most AEs with erdafitinib manageable with dose modification and best supportive care
– Therapy d/c rates 8.1% wit erdafitinib and 13.4% with chemo

When managing possible AEs with erda, what would you do next for this patient…
67 years old Male s/p 1L EV + P
+ FGFR3 alteration
Therapy with 2L erdafitinib
Serum phosphate 8.0 mg/dL
– Continue Current Dose
– Cut Dose in Half
– Withhold dose
– Permanently d/c
For phosphate of 8.0 mg/dL, withhold erdafitinib and restart once phosphate <5.5 mg/dL
Erdafitinib=good option for patients with susceptible FGFR3 alt
– What about patients who progress after 1L therapy and no FGFR3alt?
– How does the changing 1L therapy landscape with EV+P affect choice of 2L therapy?
What about T-DXd in mUC?
1st tumor agnostic ADC with FDA approval for Drug-refractory HER2+ IHC3+ Cas
From DESTINY-PanTumor02
– 16 patients with HER2 IHC3+ mUC
– 56.3% ORR, mPFS 7.4mo, mOS 13.4mo
– No significant neuropathy
AEs: pneumonitis, neutropenia, N/V, left ventricular dysfunction
Sacituzumab govitecan(SG) an ADC
Trop-2 An active agent in mUC but failed to show stat. significant longer OS over taxane or vinflunine (VIN) in TROPiCS-04
– FDA approval with drawn~Oct 2024
Let’s look original approval and possible reasons for a negative P3 trial
TROPHYU01 Cohorts 1,2,3
1. 113 patients who progrs’d after plat chemo+ICI
– Notable efficacy compared to hist cntls
– Led to accel approval 2021
2. 38 cisplatin-inelig patients s/p ICI therapy
ORR=32%
3. 41 patients who progrs’d after plat chemo with 2L SG+pembro
– ORR=41%
– mPFS=5.3m
– mOS=12.7m
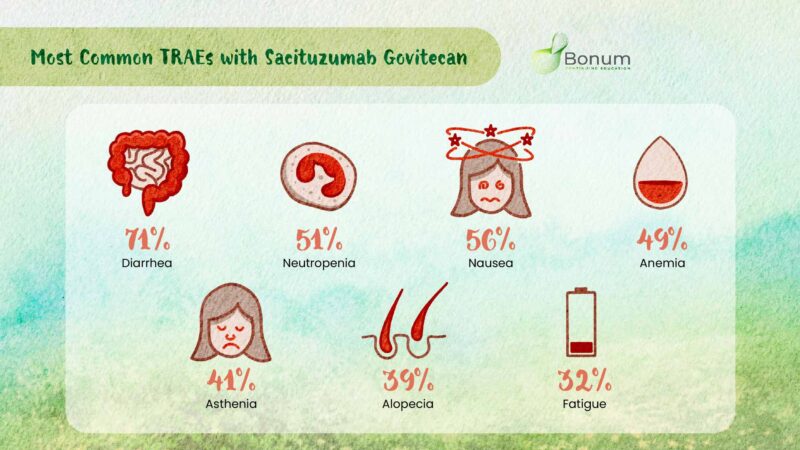
TROPHY-U-01 Cohort 3 trial safety of sacituzumab govitecan
TRAEs led to
– SG interruptions in 46%
– SG dose reduction in 39%
– SG d/c in 15%
TROPiCS-04
Ph3 RCT SG vs chemo in pretx mUC
No significant improvement in OS with SG compared to taxane or VIN
mOS: SG 10.3 vs chemo 9.0mo
(HR:0.86; 95% CI:0.73–1.02; p=0.087)
Grade ≥3 TRAEs (SG): Neutropenia(35%; FN 12%), diarrhea (15%) G5
AEs: SG 7% (16 inf with neutropenia), chemo 2%
Uromigos thoroughly addressed of sacitizumab govitecan TROPiCS-04 findings presented at ESMO Asia
Why TROPiCS4 negative for OS?
Hard to est, consider:
– Late-line,hvly pretherapy pop
– Ltd primary G-CSF prophy in SG arm(~20%)
~5% patients randomized to control never rec’d therapy(~2% in SG arm)
– NO biomarker selection(UGT1A1 gene polym→tox?)
~20% in each arm rec’d salvage EV(confounding)
SUMMARY
- 2L and later line therapies in mUC
- Erdafitinib FDA-approved with FGFR3 alt after prior therapy
- NCCN guidelines updated Jan 2025
- Ongoing trials for SG in different clinical scenarios
- T-DXd for HER2 IHC3+ (based on gastric cancer scoring algorithm)
74 y/o Female s/p gem/ + cisplatin Avelumab maintenance x12m
No FGFR3 alterations, HER2 IHC 0
New liver mets
What would you do next?
– Enfortumab Vedotin
– Erdafitinib
– Nivolumab
– Pembrolizumab
When managing possible AEs with erda, what would you do next for this patient…
67 years old Male s/p 1L EV + P
+ FGFR3 alteration
Therapy with 2L erdafitinib
Serum phosphate 8.0 mg/dL
– Continue Current Dose
– Cut Dose in Half
– Withhold dose
– Permanently d/c.”
Petros Grivas, Clinical Director of the Genitourinary Cancers Program at the University of Washington, also shared a post on X:
“Best practices for 2L+ therapy Metastatic Urothelial Carcinoma with Tian Zhang and me …assuming EV+P 1L
FGFR3 alts: THOR/erda
Trop-2: sacituzumab govitecan
HER2 IHC 3+: T-DXd Support by edu grants: Astellas, Gilead Sci, Merck, Seagen CME, Bonum CE
What’s your specialty?
– Medical Oncology
– Surgical Oncology
– Other MD/DO
– APP, RN, PharmD, Other HCP
COI and CME info
1. Answer Pre-survey
2. Review
3. claim CME

mUC SOC changing rapidly
EV+P in 1L
What does it mean for subsequent therapy?
68 years old Female with mUC s/p EV + P, on pembro monotherapy x12m (d/ced EV) FGFR3-TACC3 fusion + and cisplatin eligible
New liver mets
In your practice, what would you use next?
– Erdafinitinib
– Gem+carbo
– Gem+cisplastin
Chemo-naive cisplatin eligible patients
– Post-1L IO monotherapy
– 2L gem+cisplatin (or ddMVAC) pref’d
– Erda for FGFR3 mut/fusion (THOR level 1 evidence for erda post-IO)
– Cisplatin ineligible patients can get gem+carbo or EV (or erda if FGFR3+ alt)
Let’s consider a patient case…
74 years old Female s/p gem/ + cisplatin
– Avelumab maintenance x12m
– No FGFR3 alterations, HER2 IHC 0
– New liver mets
What would you do next?
– Enfortumab Vedotin
– Erdafitinib
– Nivolumab
– Pembrolizumab
Preferred 2L therapy for cisplatin-ineligible pts after 1L ICI monotherapy
EV or gem + carbo or erdafitinib (if FGFR alt+)
Pembro alone is one of2L therapy options for pts who progress after 1L platinum-based chemo and have not received ICI
Other 2L options(depending on prior therapy):
– Erda for FGFR3 alterations
– EV monotherapy
Taxane if no access to
– Nivo or avelumab if no access to pembro + ICI naïve
– T-DXd for HER2 3+ IHC (gastric Ca scoring)
– Sacituzumab govitecan (FDA indication withdrawn)
Atezolizumab EMA approved as monotherapy
– 1L in cisplatin-ineligible + PD-L1 +ve
– Based on IMvigor 210 and 130 trials
IMvigor 130:
– mPFS significantly longer in atezo+plat/Gem vs plat/Gem alone
– mOS not sign. longer in atezo grps vs plat/Gem alone

IMvigor130 safety results
– Fewer AEs
– withdrawal of any agent in atezo only group
– Most common TRAEs mainly related to chemo
1. Anemia
2. Neutropenia
3. Thrombocytopenia
Even with pembro 24 mo PFS rate in KEYNOTE-045 was 12.4%
Patients likely to need subsequent line therapy NCCN guidelines
RE: next treatment?
Options (if not given prior):
– EV
– Erda for FGFR3 alt
– T-DXd for HER2 IHC 3+ (gastric Ca scoring)
– Saci(indication withdrawn, still in NCCN guidlines)
Erdafitinib
– Jan 19, 2024 FDA regular approval
– Patients with mUC and FGFR3alt with progression after 1L therapy
– based on THOR1 trial of pts previously treated with PD-1/PD-L1 ICI
– Not for patients with no prior PD-1/PD-L1 if ICI eligible
Erdafitinib cont.
– mOS and mPFS significantly in erdafitinib group vs taxane or vinflunine
– THOR1 trial efficacy results

Erda. Safety
– Grade ≥3 TRAEs occurred in 45.9% with erdafitinib and 46.4% with chemo
– Most AEs with erdafitinib manageable with dose modification and best supportive care
– Therapy d/c rates 8.1% wit erdafitinib and 13.4% with chemo

When managing possible AEs with erda, what would you do next for this patient…
67 years old Male s/p 1L EV + P
+ FGFR3 alteration
Therapy with 2L erdafitinib
Serum phosphate 8.0 mg/dL
– Continue Current Dose
– Cut Dose in Half
– Withhold dose
– Permanently d/c
For phosphate of 8.0 mg/dL, withhold erdafitinib and restart once phosphate <5.5 mg/dL
Erdafitinib=good option for patients with susceptible FGFR3 alt
– What about patients who progress after 1L therapy and no FGFR3alt?
– How does the changing 1L therapy landscape with EV+P affect choice of 2L therapy?
What about T-DXd in mUC?
1st tumor agnostic ADC with FDA approval for Drug-refractory HER2+ IHC3+ Cas
From DESTINY-PanTumor02
– 16 patients with HER2 IHC3+ mUC
– 56.3% ORR, mPFS 7.4mo, mOS 13.4mo
– No significant neuropathy
AEs: pneumonitis, neutropenia, N/V, left ventricular dysfunction
Sacituzumab govitecan(SG) an ADC
Trop-2 An active agent in mUC but failed to show stat. significant longer OS over taxane or vinflunine (VIN) in TROPiCS-04
– FDA approval with drawn~Oct 2024
Let’s look original approval and possible reasons for a negative P3 trial
TROPHYU01 Cohorts 1,2,3
1. 113 patients who progrs’d after plat chemo+ICI
– Notable efficacy compared to hist cntls
– Led to accel approval 2021
2. 38 cisplatin-inelig patients s/p ICI therapy
ORR=32%
3. 41 patients who progrs’d after plat chemo with 2L SG+pembro
– ORR=41%
– mPFS=5.3m
– mOS=12.7m
TROPHY-U-01 Cohort 3 trial safety of sacituzumab govitecan
TRAEs led to
– SG interruptions in 46%
– SG dose reduction in 39%
– SG d/c in 15%

TROPiCS-04 Ph3
RCT SG vs chemo in pretherapy mUC
No significant improvement in OS with SG compared to taxane or VIN
mOS: SG 10.3 vs chemo 9.0mo
(HR:0.86; 95% CI:0.73–1.02; p=0.087)
Grade ≥3 TRAEs (SG): Neutropenia(35%; FN 12%), diarrhea (15%) G5
AEs: SG 7% (16 inf with neutropenia), chemo 2%
Uromigos thoroughly addressed of sacitizumab govitecan TROPiCS-04 findings presented at ESMO Asia
Why TROPiCS4 negative for OS?
Hard to est, consider:
– Late-line,hvly pretherapy pop
– Ltd primary G-CSF prophy in SG arm (~20%)
~5% patients randomized to control never rec’d therapy (~2% in SG arm)
– No biomarker selection (UGT1A1 gene polym→tox?)
~20% in each arm rec’d salvage EV (confounding)
SUMMARY
- 2L and later line therapies in mUC
- Erdafitinib FDA-approved with FGFR3 alt after prior therapy
- NCCN guidelines updated Jan 2025
- Ongoing trials for SG in different clinical scenarios
- T-DXd for HER2 IHC3+ (based on gastric cancer scoring algorithm)
74 y/o Female s/p gem/ + cisplatin Avelumab maintenance x12m
No FGFR3 alterations, HER2 IHC 0
New liver mets
What would you do next?
– Enfortumab Vedotin
– Erdafitinib
– Nivolumab
– Pembrolizumab
When managing possible AEs with erda, what would you do next for this patient…
67 years old Male s/p 1L EV + P
+ FGFR3 alteration
Therapy with 2L erdafitinib
Serum phosphate 8.0 mg/dL
– Continue Current Dose
– Cut Dose in Half
– Withhold dose
– Permanently d/c.”
Further Reading:
Enfortumab Vedotin (Padcev): Uses in Cancer, Side Effects, Dosage, Expectations, and More
