Raffaele Colombo, Principal Scientist at Zymeworks, shared on X/Twitter:
106 auristatin Antibody Drug Conjugates (ADCs) have reached clinic development, of which two-thirds (= 71 ADC) with Monomethyl auristatin E (MMAE) as payload! Here the breakdown of the MMAE ADCs:
- 4 approved by FDA
- 1 approved by the National Medical Products Administration in China
- 41 in clinical development
- 25 discontinued
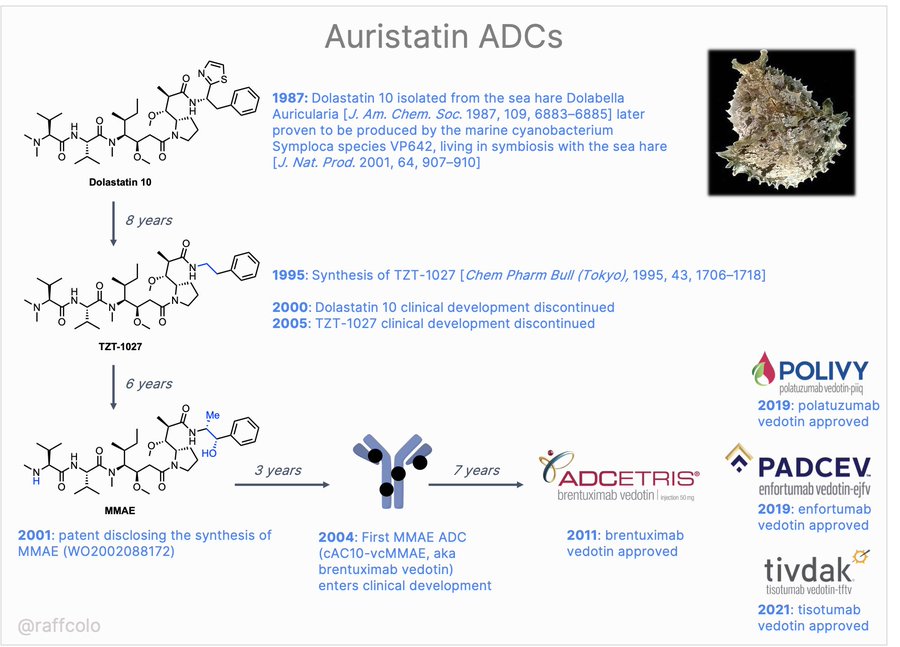
The MMAE history:
Source: Raffaele Colombo/Twitter