Elad Sharon, Clinical and Translational Director of Dana-Farber Cancer Institute, shared a post by FDA Oncology on X:
“Absolutely, the right message to send… Contribution of effect is an under appreciated and essential part of drug development.
I don’t even think the perioperative period is the most essential place to start… but you gotta start somewhere.”
Quoting FDA Oncology’s post:
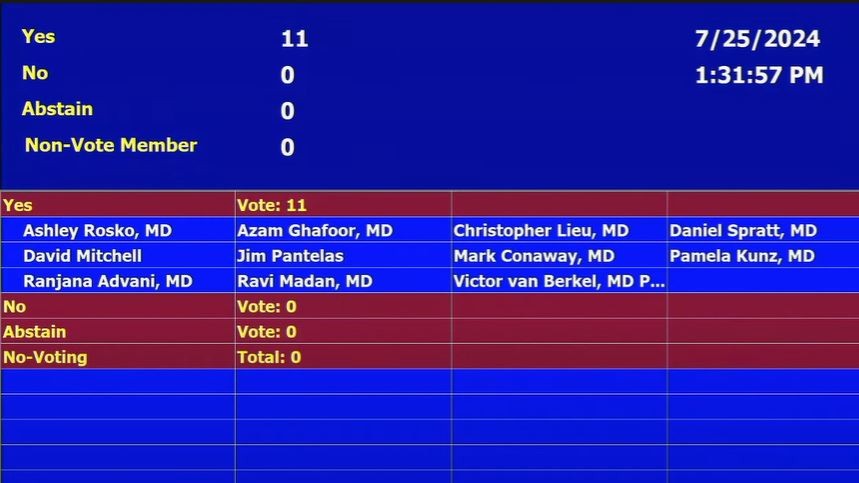
“Oncologic Drugs Advisory Committee votes 11 to 0 in favor of FDA requiring that new trial design proposals for perioperative regimens for resectable NSCLC include adequate within trial assessment of contribution of treatment phase.”
Link to video.
Source: Elad Sharon/X and FDA Oncology/X