Neoadjuvant treatment for high-risk HR+/HER2- early breast cancer (EBC) remains challenging, with recurrence risks persisting despite chemotherapy (CHT) and endocrine therapy (ET).
The TOT-HER3 trial showed that a single dose of HER3-DXd, a HER3-directed antibody-drug conjugate, led to clinical response, increased immune infiltration, and suppression of proliferation, with a manageable safety profile in HR+/HER2- EBC patients (Prat et al. ESMO Breast 2022). These findings informed the design of the VALENTINE trial, investigating HER3-DXd in the neoadjuvant setting.
The Study was published on May 2023 on ESMO Open. Resents of Trial were presented at SABCS 2024.
155TiP A randomised phase II trial of neoadjuvant multi-agent chemotherapy (CHT) OR patritumab deruxtecan (HER3-DXd; U3-1402) +/- endocrine therapy (ET) for high-risk hormone receptor-positive (HR+/HER2-) early breast cancer (EBC): SOLTI-2103 VALENTINE trial
Authors: M. Oliveira, T. Pascual, G. Villacampa, M. Munoz, A. Perello Martorell, M.E. Perez Lopez, K. Amillano Parraga, X. González Farre, C. Martinez Vila, P. Tolosa Ortega, M. Borrell Puy, M. Margeli Vila, J.M. Cejalvo, Y. Zheteyava, D.W. Sternberg, P.D. Fan, A. Santhanagopal, R. Sanchez Bayona, J.M. Ferrero-Cafiero, A. Prat

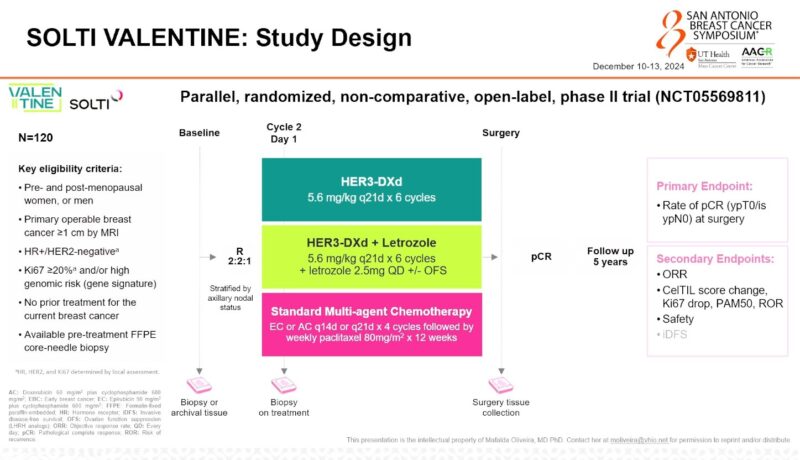
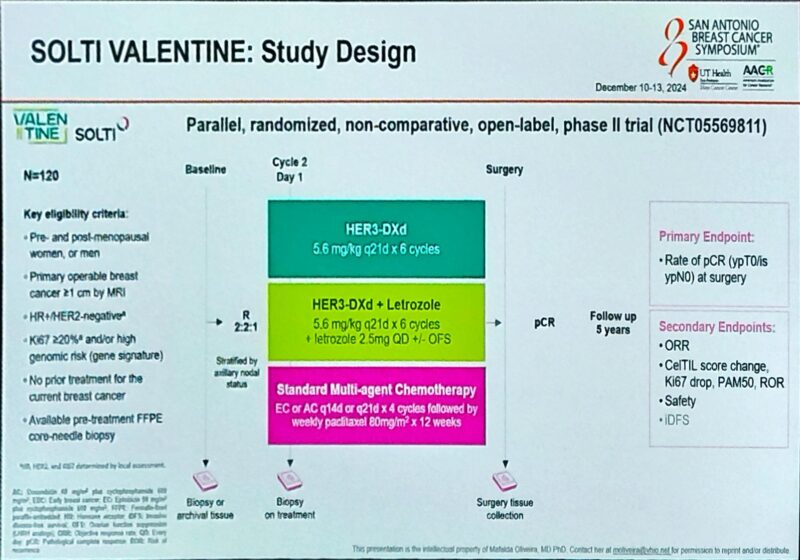
Trial Design
The VALENTINE trial is a parallel, exploratory, three-arm, randomised, open-label study designed to assess the clinical benefit and biological effects of HER3-DXd as a neoadjuvant treatment for primary operable HR+/HER2-negative breast cancer. The trial targets patients with Ki67 IHC ≥ 20% and/or high genomic risk, defined by gene signature. A total of 120 treatment-naïve patients will be randomly assigned in a 2:2:1 ratio to one of three groups:
- HER3-DXd (5.6 mg/kg) every 21 days for 6 cycles.
- HER3-DXd plus daily letrozole (LET), with or without LHRH analogs, every 21 days for 6 cycles.
- Standard of care chemotherapy (CHT) consisting of 4 cycles of EC/AC (epirubicin 90 mg/m2 or doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2) every 14/21 days, followed by weekly paclitaxel (80 mg/m2) for 12 weeks.
The primary endpoint is the rate of pathological complete response (ypT0/is ypN0) at surgery. Baseline, on-treatment (C2D1), and surgical specimens will be collected for molecular characterization, including ERBB3 gene expression, HER3 IHC, CelTIL change, and PAM50 subtypes. Additional samples will be collected for pharmacokinetic, genomic, and circulating biomarkers.
Secondary endpoints include the rate of residual cancer burden, overall response rate, invasive disease-free survival at 3 and 5 years, and safety. Both interim and final analyses are planned.
About the Author of the Study – Mafalda Oliveira

SOLTI
“Today, SOLTI-2103 VALENTINE was presented in SABCS24 by Dr. Mafalda Oliveira!”

Sara Tolaney
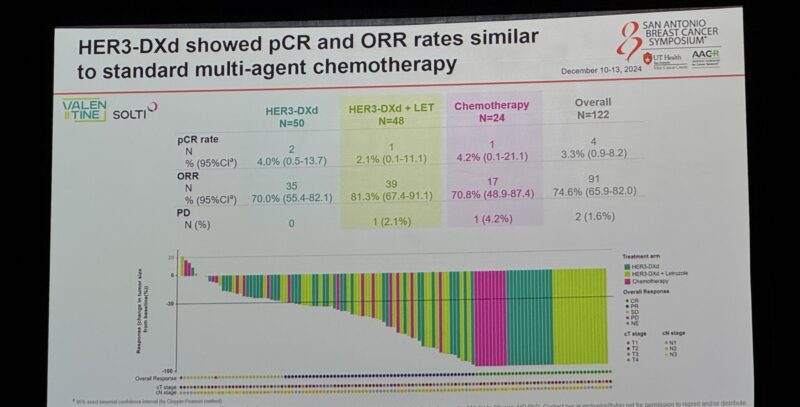
“VALENTINE: Preop HER3DXd in ER+ BC Ki67≥20% or high genomic assay HER3DXd +/- letrozole vs A(or E) -CT n=120
pCR: 4% vs 2.1% vs 4.2%.”
Gaia Griguolo
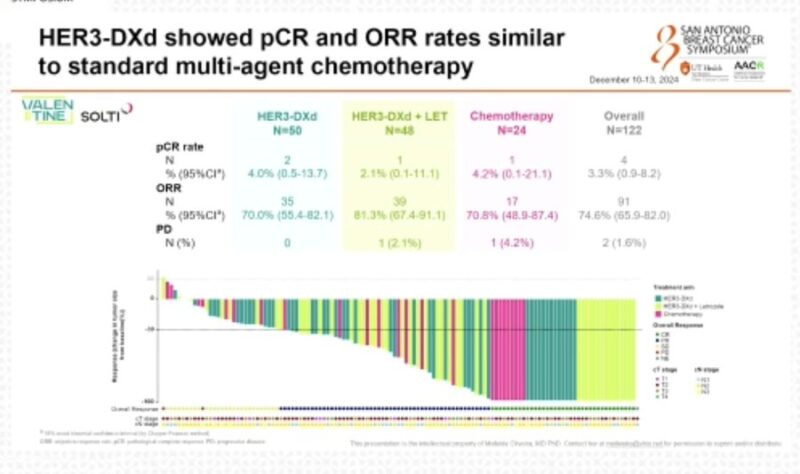
“Great presentation of SOLTI VALENTINE trial by Mafalda Oliveira at SABCS24.
HER3-DXd showed similar pCR and ORR rates to standard CT with better tolerability profile.”
Amol Akhade
“Encouraging results with neo adjuvant HER3-Dxd with letrozole in neoadjuvant Hr positive and her2 negative EBC .
We need to see if it changes EFS on longer follow up Solti Valentine trial.”

Puneet Singh
“Mafalda Oliveira presents SOLTI VALENTINE: neoadj randomized study of HER3-DXd, an ADC, in high-risk early stage HR+ breast cancer – comparable pCR rate to chemo however still low rates of pCR at SABCS24.”
José Sandoval
“SOLTI Valentine: HER3-DXd +/- ET or Chemo in HR+/HER2- high risk EBC.
pCR rates similar.
Yet, it’s a randomised noncomparative trial…is it legit to compare? HER3-DXd is effective. IMO, we’ll need better EFS (or better QoL) for routine use (after a bigger trial).”