Bhavin Vadodariya, Consultant Surgical Oncologist at SSO Cancer Hospital and Clinics, PSM Hospital, and Shankus Hospitals, shared a post on LinkedIn:
“Paradigm Shift in Head and Neck Cancer: ASCO 2025 Sets a New Standard
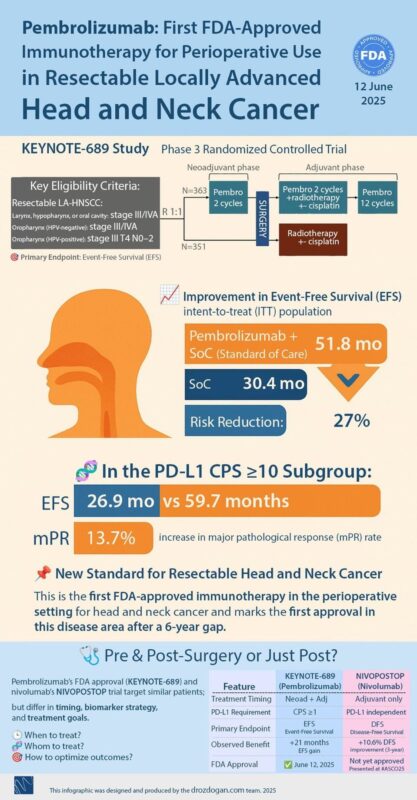
For two decades, the curative management of resectable head and neck cancer remained unchanged. That changed at ASCO 2025.
KEYNOTE-689 introduced perioperative pembrolizumab – 2 doses before surgery, followed by chemoradiation and 12 cycles post-op. This led to a dramatic improvement in event-free survival (51.8 vs. 30.4 months), a 13.7% rise in major pathological response, and a 27% risk reduction. In patients with PD-L1 CPS ≥10, outcomes were even more compelling.
NIVOPOSTOP, on the other hand, showed that adding adjuvant nivolumab after chemoradiation in high-risk surgical cases improved 3-year DFS from 52.5% to 63.1%, irrespective of PD-L1 expression – making it more adaptable in low-resource settings.
In my practice, this means:
Sharper patient selection- T3/T4a tumors, ECE, R1 resections, ECOG 0–1, <75 yrs
PD-L1 testing as routine,
Immunotherapy integrated tumor board planning.
Integrating perioperative immunotherapy into head and neck cancer care demands a shift in how we select and manage patients – factoring in tumor biology, immune markers like PD-L1 CPS, and performance status.
While pembrolizumab-based protocols will require advanced profiling and significant cost (₹15–20 lakhs/year), the NIVOPOSTOP approach offers a more practical, PD-L1-independent option with fewer doses. This will push tumor boards to evolve, with discussions now including Immunotherapy sequencing and timing.
Yet, in India, access remains the biggest hurdle – making pragmatic, cost-aware implementation the key to ensuring these breakthroughs benefit patients beyond the headlines.”
More posts featuring Bhavin Vadodariya.
