Raffaele Colombo, Associate Director of Medicinal Chemistry at Zymeworks Inc., shared a post on X:
“Dato-DXd now approved for patients with EGFR-mutated NSCLC after EGFR-directed and platinum-based therapies!
Accelerated approval based on TROPION-Lung05 and TROPION-Lung01
ORR was 45% (95% CI: 35, 54) and median DOR was 6.5 months (95% CI: 4.2, 8.4)”

Later Raffaele Colombo shared this post, adding:
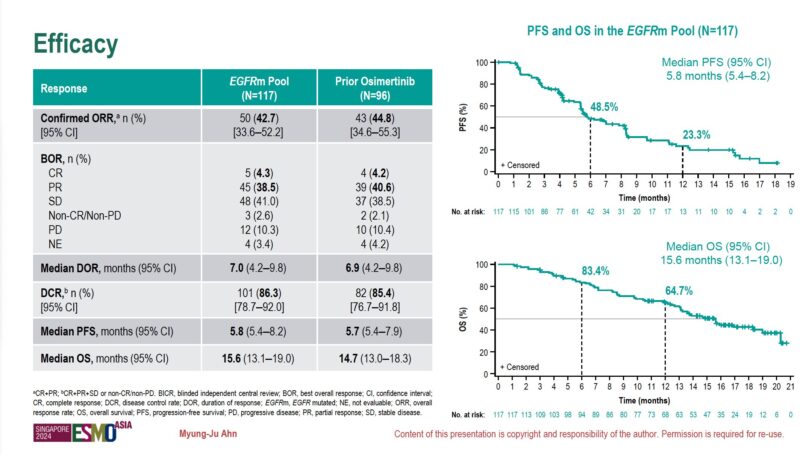
“Pooled analysis of EGFR-mutant NSCLC from TROPION-Lung05 and TROPION-Lung01 was presented at ESMOASIA24.”
Later he also added:
“I didn’t know dato-DXd was already approved in Russia for EGFR-mutant NSCLC patients…3 months ahead of the U.S.!”

More posts featuring Raffaele Colombo.
