Mark Lewis shared a post by FDA Oncology on X, adding the following: .
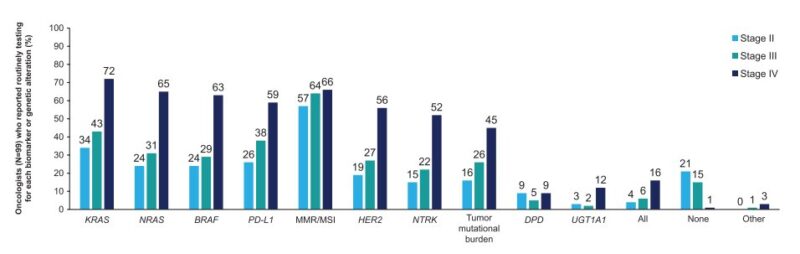
“We must hold oncologists treating colorectal cancer to the same biomarker testing standards that we would expect in mgmt of breast cancer
It is UNACCEPTABLE to treat mCRC without knowing:
- kras/nras/braf
- MMR/MSI
- her2
Yet fewer than 2/3rds of patients get this testing!”
Quoting FDA Oncology’s post:
“FDA grants accelerated approval to adagrasib with cetuximab for KRAS G12C-mutated colorectal cancer.”
Read further.
Source: Mark Lewis/X and FDA Oncology/X
Mark Lewis is the Director of Gastrointestinal Oncology at Intermountain Healthcare in Utah, the Co-Chair of adolescent and young adult (AYA) oncology in the SWOG cooperative group, and the Vice President of American Multiple Endocrine Neoplasia Support. Dr. Lewis is also a well-known patient advocate and social media influencer.
