Ivan R. Gonzalez, Medical Oncologist at Comprehensive Oncology Center, Hospital Angeles Puebla, shared a post on X:
”Project Orbis: A global initiative to accelerate the review of cancer drugs in the US, Canada, and the UK (2019-2023) reveals:
- 33% of FDA approvals through Orbis.
- Overall survival: 4.1 months (Orbis) vs. 2.7 months (others).
- Reimbursements: 100% (Scotland), 40% (England), 90% (Canada, conditional).
- Access times significantly increased.
- Average monthly cost: $20,000 USD.
Spending on medications surpasses the rate of new cases. It’s crucial to evaluate benefits and costs for a comprehensive global health impact. Project Orbis is expanding, but a deeper understanding of its implications is needed.”
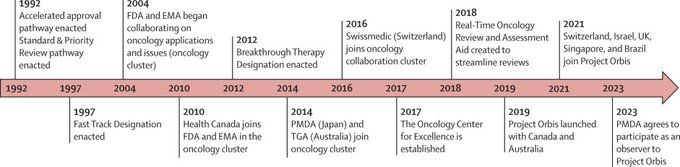
Source: Ivan R. Gonzalez/X
